Persistent glucose consumption under antibiotic treatment protects bacterial community
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Antibiotics typically induce major physiological changes in bacteria. However, their effect on nutrient consumption remains unclear. Here we found that Escherichia coli communities can sustain normal levels of glucose consumption under a broad range of antibiotics. The community-living resulted in a low membrane potential in the bacteria, allowing slow antibiotic accumulation on treatment and better adaptation. Through multi-omics analysis, we identified a prevalent adaptive response characterized by the upregulation of lipid synthesis, which substantially contributes to sustained glucose consumption. The consumption was maintained by the periphery region of the community, thereby restricting glucose penetration into the community interior. The resulting spatial heterogeneity in glucose availability protected the interior from antibiotic accumulation in a membrane potential-dependent manner, ensuring rapid recovery of the community postantibiotic treatment. Our findings unveiled a community-level antibiotic response through spatial regulation of metabolism and suggested new strategies for antibiotic therapies. Community-living promotes bacterial adaptation to antibiotics, allowing persistent glucose consumption and maintenance of spatial heterogeneity in the community, ensuring rapid recovery of the community postantibiotic treatment.

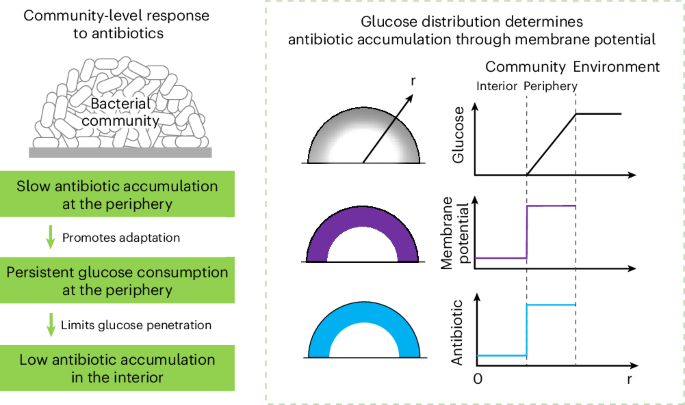
抗生素治疗下的持续葡萄糖消耗保护细菌群落
抗生素通常会引起细菌的重大生理变化。然而,它们对营养物质消耗的影响仍不清楚。在这里,我们发现大肠埃希氏菌群落能在多种抗生素作用下维持正常水平的葡萄糖消耗。群落生活导致细菌的膜电位较低,从而使抗生素在处理过程中积累缓慢,适应性更好。通过多组学分析,我们发现了一种普遍的适应性反应,其特点是脂质合成的上调,这大大促进了持续的葡萄糖消耗。这种消耗由群落外围区域维持,从而限制了葡萄糖向群落内部的渗透。由此产生的葡萄糖可用性的空间异质性以膜电位依赖的方式保护了内部免受抗生素的积累,确保了抗生素治疗后群落的快速恢复。我们的研究结果揭示了一种通过空间调节新陈代谢的群落级抗生素反应,并提出了抗生素疗法的新策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


