Docking a flexible basket onto the core of the nuclear pore complex
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
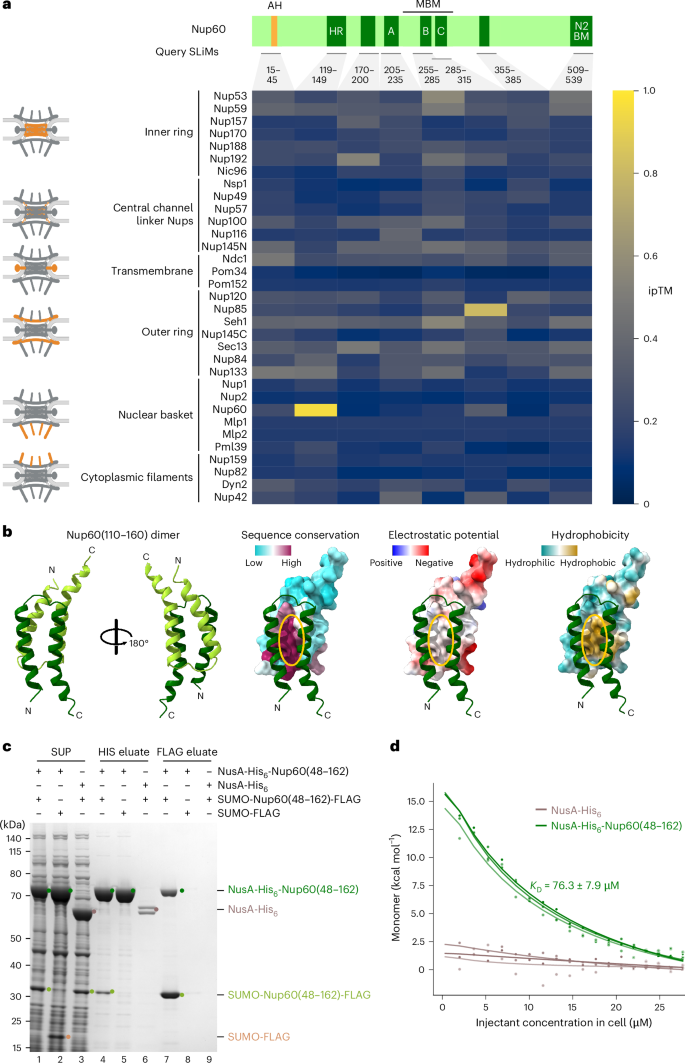
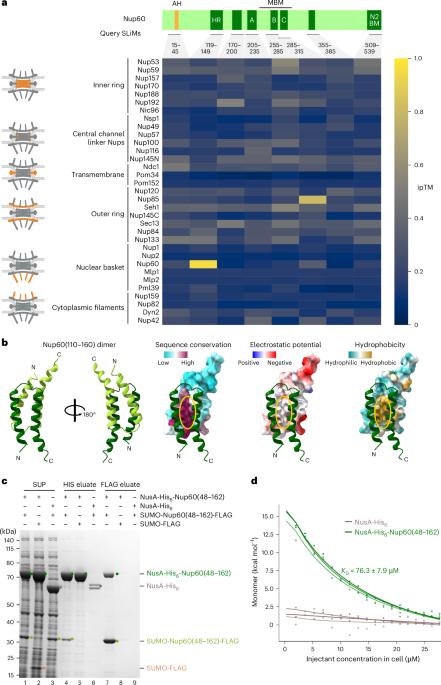
The nuclear basket attaches to the nucleoplasmic side of the nuclear pore complex (NPC), coupling transcription to mRNA quality control and export. The basket expands the functional repertoire of a subset of NPCs in Saccharomyces cerevisiae by drawing a unique RNA/protein interactome. Yet, how the basket docks onto the NPC core remains unknown. By integrating AlphaFold-based interaction screens, electron microscopy and membrane-templated reconstitution, we uncovered a membrane-anchored tripartite junction between basket and NPC core. The basket subunit Nup60 harbours three adjacent short linear motifs, which connect Mlp1, a parallel homodimer consisting of coiled-coil segments interrupted by flexible hinges, and the Nup85 subunit of the Y-complex. We reconstituted the Y-complex•Nup60•Mlp1 assembly on a synthetic membrane and validated the protein interfaces in vivo. Here we explain how a short linear motif-based protein junction can substantially reshape NPC structure and function, advancing our understanding of compositional and conformational NPC heterogeneity. Stankunas and Köhler define how the nucleoplasmic portion of the nuclear pore complex (NPC), the basket, docks onto the NPC core by integrating AlphaFold-based interaction screens, electron microscopy, and membrane-templated reconstitutions.
将柔性篮对接到核孔复合体的核心上
核篮子附着在核孔复合体(NPC)的核质侧,将转录与 mRNA 质量控制和输出结合在一起。核篮子通过吸引独特的 RNA 蛋白相互作用组,扩大了酿酒酵母中 NPC 亚群的功能范围。然而,篮子如何与 NPC 核心对接仍是未知数。通过整合基于 AlphaFold 的相互作用筛选、电子显微镜和膜诱导重组,我们发现了篮子和 NPC 核心之间的膜锚定三方连接。篮子亚基 Nup60 包含三个相邻的短线性基团,它们连接着 Mlp1(由柔性铰链中断的盘绕段组成的平行同源二聚体)和 Y 复合物的 Nup85 亚基。我们在合成膜上重建了 Y-复合体-Nup60-Mlp1 组装,并在体内验证了蛋白质界面。在这里,我们解释了基于短线性基团的蛋白质连接是如何大幅重塑 NPC 结构和功能的,从而推进了我们对 NPC 组成和构象异质性的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


