The role of biomolecular condensates in protein aggregation
IF 38.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
There is an increasing amount of evidence that biomolecular condensates are linked to neurodegenerative diseases associated with protein aggregation, such as Alzheimer’s disease and amyotrophic lateral sclerosis, although the mechanisms underlying this link remain elusive. In this Review, we summarize the possible connections between condensates and protein aggregation. We consider both liquid-to-solid transitions of phase-separated proteins and the partitioning of proteins into host condensates. We distinguish five key factors by which the physical and chemical environment of a condensate can influence protein aggregation, and we discuss their relevance in studies of protein aggregation in the presence of biomolecular condensates: increasing the local concentration of proteins, providing a distinct chemical microenvironment, introducing an interface wherein proteins can localize, changing the energy landscape of aggregation pathways, and the presence of chaperones in condensates. Analysing the role of biomolecular condensates in protein aggregation may be essential for a full understanding of amyloid formation and offers a new perspective that can help in developing new therapeutic strategies for the prevention and treatment of neurodegenerative diseases. Biomolecular condensates help organize cell components under normal conditions but can also be involved in pathological protein aggregation when condensate proteins carry mutations or under stress conditions. This Review discusses the possible mechanisms behind such aggregation processes that potentially lead to neurodegenerative diseases.
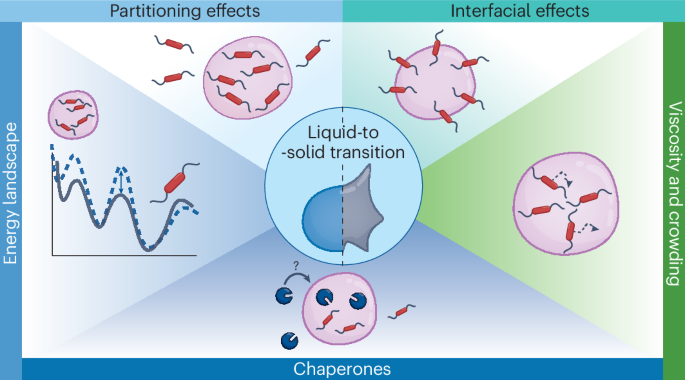
生物分子凝聚体在蛋白质聚集中的作用。
越来越多的证据表明,生物分子凝聚物与阿尔茨海默病和肌萎缩性脊髓侧索硬化症等与蛋白质聚集有关的神经退行性疾病有关,但这种联系的内在机制仍然难以捉摸。在这篇综述中,我们总结了凝聚态与蛋白质聚集之间可能存在的联系。我们既考虑了相分离蛋白质的液固转换,也考虑了蛋白质在宿主凝聚物中的分区。我们区分了冷凝物的物理和化学环境可影响蛋白质聚集的五个关键因素,并讨论了它们在生物分子冷凝物存在下的蛋白质聚集研究中的相关性:增加蛋白质的局部浓度、提供独特的化学微环境、引入蛋白质可定位的界面、改变聚集途径的能量景观以及冷凝物中存在伴侣。分析生物分子凝聚物在蛋白质聚集过程中的作用可能对全面了解淀粉样蛋白的形成至关重要,并提供了一个新的视角,有助于开发预防和治疗神经退行性疾病的新疗法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature reviews. Chemistry
Chemical Engineering-General Chemical Engineering
CiteScore
52.80
自引率
0.80%
发文量
88
期刊介绍:
Nature Reviews Chemistry is an online-only journal that publishes Reviews, Perspectives, and Comments on various disciplines within chemistry. The Reviews aim to offer balanced and objective analyses of selected topics, providing clear descriptions of relevant scientific literature. The content is designed to be accessible to recent graduates in any chemistry-related discipline while also offering insights for principal investigators and industry-based research scientists. Additionally, Reviews should provide the authors' perspectives on future directions and opinions regarding the major challenges faced by researchers in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


