Extremely acidic proteomes and metabolic flexibility in bacteria and highly diversified archaea thriving in geothermal chaotropic brines
IF 13.9
1区 生物学
Q1 ECOLOGY
引用次数: 0
Abstract
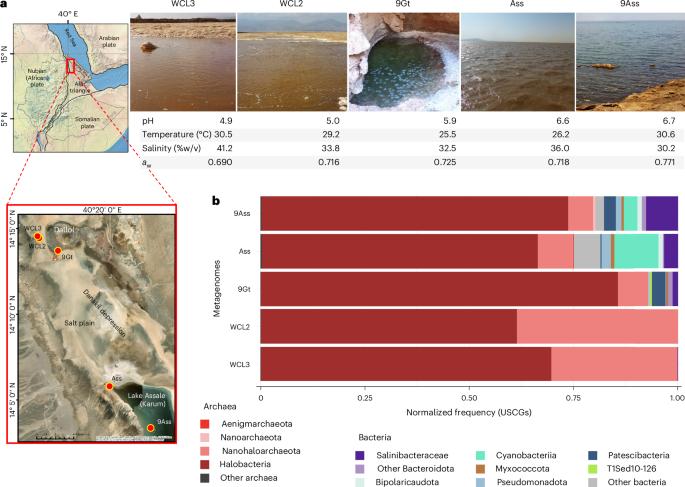
Few described archaeal, and fewer bacterial, lineages thrive under salt-saturating conditions, such as solar saltern crystallizers (salinity above 30% w/v). They accumulate molar K+ cytoplasmic concentrations to maintain osmotic balance (‘salt-in’ strategy) and have proteins adaptively enriched in negatively charged acidic amino acids. Here we analysed metagenomes and metagenome-assembled genomes from geothermally influenced hypersaline ecosystems with increasing chaotropicity in the Danakil Depression. Normalized abundances of universal single-copy genes confirmed that haloarchaea and Nanohaloarchaeota encompass 99% of microbial communities in the near-life-limiting conditions of the Western-Canyon Lakes. Danakil metagenome- and metagenome-assembled-genome-inferred proteomes, compared with those of freshwater, seawater and solar saltern ponds up to saturation (6–14–32% salinity), showed that Western-Canyon Lake archaea encode the most acidic proteomes ever observed (median protein isoelectric points ≤4.4). We identified previously undescribed haloarchaeal families as well as an Aenigmatarchaeota family and a bacterial phylum independently adapted to extreme halophily. Despite phylum-level diversity decreasing with increasing salinity–chaotropicity, and unlike in solar salterns, adapted archaea exceedingly diversified in Danakil ecosystems, challenging the notion of decreasing diversity under extreme conditions. Metabolic flexibility to utilize multiple energy and carbon resources generated by local hydrothermalism along feast-and-famine strategies seemingly shapes microbial diversity in these ecosystems near life limits. Metagenome analysis of microbial communities from the Danakil Depression shows that haloarchaea and Nanohaloarchaeota dominate these life-limiting salt-saturated conditions and that these archaea have the most acidic proteomes observed so far.
在地热混沌盐水中生长的细菌和高度多样化古菌的极酸性蛋白质组和代谢灵活性。
在盐饱和的条件下,如在日晒盐霜结晶器(盐度高于 30% w/v)中,很少有已描述的古细菌,也很少有细菌,能茁壮成长。它们积累摩尔 K+ 细胞质浓度以维持渗透平衡("盐进 "策略),并使蛋白质适应性地富含带负电荷的酸性氨基酸。在这里,我们分析了来自达纳基尔洼地受地热影响的高盐生态系统的元基因组和元基因组组装的基因组,这些生态系统的混沌倾向性不断增加。通用单拷贝基因的归一化丰度证实,在西部峡谷湖泊接近生命极限的条件下,卤代古细菌和纳米卤代古细菌占微生物群落的99%。达纳基尔元基因组和元基因组组装的基因组推断出的蛋白质组,与淡水、海水和日照盐池中达到饱和(6-14-32% 盐度)的蛋白质组进行了比较,结果表明西峡谷湖古细菌编码了迄今为止观察到的酸性最强的蛋白质组(蛋白质等电点中位数≤4.4)。我们发现了以前未曾描述过的卤代古细菌科以及一个Aenigmatarchaeota科和一个独立适应极端嗜卤的细菌门。尽管随着盐度-朝向性的增加,门一级的多样性会减少,但与日照盐碱地不同,达纳基尔生态系统中适应性古细菌的多样性超乎寻常,这对极端条件下多样性减少的观点提出了挑战。利用当地热液作用产生的多种能量和碳资源的新陈代谢灵活性,以及 "盛宴-饥饿 "策略似乎塑造了这些生态系统中接近生命极限的微生物多样性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature ecology & evolution
Agricultural and Biological Sciences-Ecology, Evolution, Behavior and Systematics
CiteScore
22.20
自引率
2.40%
发文量
282
期刊介绍:
Nature Ecology & Evolution is interested in the full spectrum of ecological and evolutionary biology, encompassing approaches at the molecular, organismal, population, community and ecosystem levels, as well as relevant parts of the social sciences. Nature Ecology & Evolution provides a place where all researchers and policymakers interested in all aspects of life's diversity can come together to learn about the most accomplished and significant advances in the field and to discuss topical issues. An online-only monthly journal, our broad scope ensures that the research published reaches the widest possible audience of scientists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


