Microbial intestinal dysbiosis drives long-term allergic susceptibility by sculpting an ILC2–B1 cell–innate IgE axis
IF 11.2
1区 医学
Q1 ALLERGY
引用次数: 0
Abstract
Background
The abundance and diversity of intestinal commensal bacteria influence systemic immunity with impact on disease susceptibility and severity. For example, loss of short chain fatty acid (SCFA)-fermenting bacteria in early life (humans and mice) is associated with enhanced type 2 immune responses in peripheral tissues including the lung.
Objective
Our goal was to reveal the microbiome-dependent cellular and molecular mechanisms driving enhanced susceptibility to type 2 allergic lung disease.
Methods
We used low-dose vancomycin to selectively deplete SCFA-fermenting bacteria in wild-type mice. We then examined the frequency and activation status of innate and adaptive immune cell lineages with and without SCFA supplementation. Finally, we used ILC2-deficient and signal transducer and activator of transcription 6 (STAT6)-deficient transgenic mouse strains to delineate the cellular and cytokine pathways leading to enhanced allergic disease susceptibility.
Results
Mice with vancomycin-induced dysbiosis exhibited a 2-fold increase in lung ILC2 primed to produce elevated levels of IL-2, -5, and -13. In addition, upon IL-33 inhalation, mouse lung ILC2 displayed a novel ability to produce high levels of IL-4. These expanded and primed ILC2s drove B1 cell expansion and IL-4–dependent production of IgE that in turn led to exacerbated allergic inflammation. Importantly, these enhanced lung inflammatory phenotypes in mice with vancomycin-induced dysbiosis were reversed by administration of dietary SCFA (specifically butyrate).
Conclusion
SCFAs regulate an ILC2–B1 cell–IgE axis. Early-life administration of vancomycin, an antibiotic known to deplete SCFA-fermenting gut bacteria, primes and amplifies this axis and leads to lifelong enhanced susceptibility to type 2 allergic lung disease.
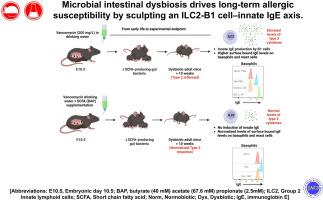
微生物肠道菌群失调通过塑造 ILC2-B1 细胞-innate IgE 轴驱动长期过敏易感性。
背景:肠道共生细菌的丰度和多样性会影响全身免疫力,并对疾病的易感性和严重程度产生影响。例如,生命早期短链脂肪酸(SCFA)发酵菌的丧失(人类和小鼠)与包括肺部在内的外周组织的 2 型免疫反应增强有关:我们的目标是揭示导致 2 型过敏性肺病易感性增强的微生物依赖性细胞和分子机制:方法:我们使用低剂量万古霉素选择性地清除野生型小鼠(Vanc-dys小鼠)体内的SCFA发酵菌。然后,我们检测了补充和未补充 SCFA 的先天性免疫细胞系和适应性免疫细胞系的频率和活化状态。最后,我们使用 ILC2 缺陷和信号转导和激活转录 6(STAT6)缺陷的转基因小鼠品系来确定导致过敏性疾病易感性增强的细胞和细胞因子途径:此外,经 IL-33 处理后,Vanc-dys 肺 ILC2 显示出产生高水平 IL-4 的新能力。这些扩增和激活的 ILC2 驱动 B1 细胞扩增,并产生依赖 IL-4 的 IgE,进而导致过敏性炎症加剧。重要的是,Vanc-dys 小鼠肺部炎症表型的增强可通过摄入 SCFA(特别是丁酸盐)而逆转:结论:SCFA 可调节 ILC2-B1cell-IgE 轴。结论:SCFA 可调节 ILC2-B1cell-IgE轴。早期服用万古霉素(一种已知会耗尽 SCFA 发酵肠道细菌的抗生素)可激发和放大该轴,并导致终生易患 2 型过敏性肺病。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
25.90
自引率
7.70%
发文量
1302
审稿时长
38 days
期刊介绍:
The Journal of Allergy and Clinical Immunology is a prestigious publication that features groundbreaking research in the fields of Allergy, Asthma, and Immunology. This influential journal publishes high-impact research papers that explore various topics, including asthma, food allergy, allergic rhinitis, atopic dermatitis, primary immune deficiencies, occupational and environmental allergy, and other allergic and immunologic diseases. The articles not only report on clinical trials and mechanistic studies but also provide insights into novel therapies, underlying mechanisms, and important discoveries that contribute to our understanding of these diseases. By sharing this valuable information, the journal aims to enhance the diagnosis and management of patients in the future.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


