Selective 1,1- and 1,2-dibromination of phenylethanes in the presence of NaBr/NaBrO3/H2SO4 as the bromination reagent†
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Selective 1,1- and 1,2-dibromination of phenylethanes by simply adjusting the reaction conditions has been developed. Mixtures of NaBr/NaBrO3/H2SO4 are employed as green bromination reagents, which can release Br2 or BrOH in situ as required without polluting the environment. Both the resulting 1,1- and 1,2-dibromoethyl arenes can be easily transformed to phenylacetylenes via elimination under basic conditions, demonstrating great potential for industrial applications.
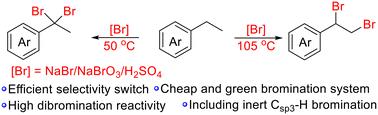
在 NaBr/NaBrO3/H2SO4 作为溴化试剂的情况下,选择性地对苯乙烷进行 1,1- 和 1,2- 二溴反应。
通过简单调整反应条件,开发出了苯乙烷的选择性 1,1- 和 1,2- 二溴化反应。NaBr/NaBrO3/H2SO4 的混合物被用作绿色溴化试剂,可根据需要就地释放出 Br2 或 BrOH,不会对环境造成污染。在碱性条件下,生成的 1,1- 和 1,2- 二溴乙基炔都能通过消去反应轻松转化为苯乙炔,显示出巨大的工业应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
The international home of synthetic, physical and biomolecular organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


