Practical method for the large-scale synthesis of 4′-chloro-2-nitrobiphenyl: A key intermediate of Boscalid
引用次数: 0
Abstract
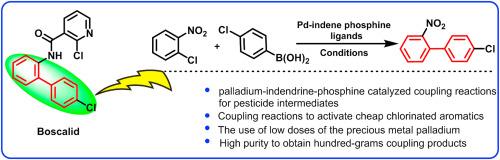
Boscalid is a pesticide with the advantages of broad spectrum bactericidal activity, high efficiency, low toxicity, and no cross-resistance with other fungicides currently available on the market. Herein, we report the synthesis of 4′-chloro-2-nitrobiphenyl, a key intermediate of Boscalid using a palladium-catalyzed Suzuki-Miyaura cross-coupling employing the 2-aryl-substituted indenyl phosphine ligand. 4′-Chloro-2-nitrobiphenyl was prepared in 94 % yield on a 100 g scale. This method allows for the industrial production of alimide and active substances bearing a biphenyl moiety.
大规模合成 4′-氯-2-硝基联苯的实用方法:Boscalid 的关键中间体
Boscalid 是一种杀虫剂,具有广谱杀菌活性、高效、低毒以及与目前市场上的其他杀菌剂无交互抗性等优点。在此,我们报告了利用 2-芳基取代的茚基膦配体,通过钯催化的铃木-宫浦交叉偶联合成 4′-氯-2-硝基联苯的过程,这是 Boscalid 的关键中间体。在 100 克的规模上,4′-氯-2-硝基联苯的制备收率为 94%。利用这种方法可以工业化生产烯丙基酰胺和含有联苯分子的活性物质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


