A novel interaction theory for the starch adsorption onto hematite surface
Abstract
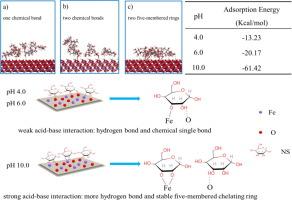
Depressant starch (NS) was generally used in hematite flotation, while the adsorption mechanism of the macromolecular polymer onto mineral surfaces remained in question. In this study, novel detection approaches and computational chemistry methods were introduced to update the widely-accepted acid-base interaction theory. Microflotation tests confirm that the hematite flotation recovery was easily depressed by NS under the acid or alkaline conditions rather than the neutral condition. Zeta potential measurement shows that NS could change the zeta potential of hematite, while the shift amplitude ranked as alkaline > acid > neutral, indicating the most suitable pH range is the alkaline condition. XPS analysis reveals that NS could chemisorbed onto Fe atoms of hematite surface via C-O groups in the whole studied pH range. It was further verified using AFM tests, in which the NS has a stronger interaction force under the alkaline environment. MDS further indicates that the interaction energy between NS and the (0 0 1) hematite surface was three times greater than others under alkaline conditions. In general, the interaction force at the interface between the hematite surface and NS was a strong chemical adsorption at the alkaline conditions while there was weak chemisorption and hydrogen bonding under the neutral or acidic conditions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


