Synthesis of a Palladium Dimer Supported by a C-Bound Trifluoroacetonate Bridge Formed by Cleavage of a Hexafluoroacetylacetonate Ligand
IF 2.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
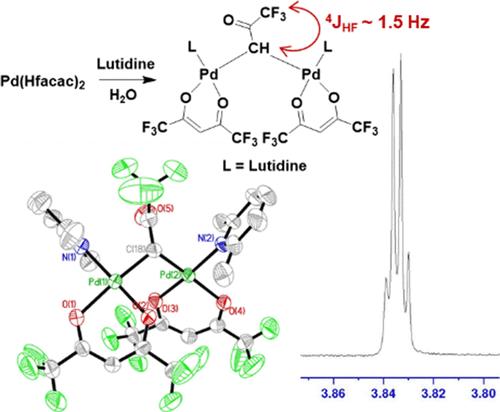
Palladium(II) hexafluoroacetylacetonate (Pd(Hfacac)2) is known to form adducts of bases, such as lutidine (2,6-dimethylpyridine). When treated with approximately 3 equiv of lutidine, Pd(Hfacac)2 yields a 1:1 complex as reported in the literature, Pd(O,O-Hfacac)(C-Hfacac)(lutidine), 1. However, when the amount of excess lutidine is increased, a new complex, 2, is formed. A single-crystal X-ray structure of 2 proves it is a rare example of a dimeric palladium complex containing two Pd(Hfacac)(lutidine) fragments bridged by a dianionic trifluoroacetonate ligand, μ-CHC(O)CF3. The formation of 2 is accompanied by a white precipitate determined to be a mixture of trans-Pd(O2CCF3)2(lutidine)2 (3), confirming the fate of the missing trifluoroacetate fragment from the cleavage of the Hfacac ligand, and [lutidinium][Hfacac] (4). Subsequent experiments revealed the determinative role that water played in this reaction. The mechanism of cleavage of the Hfacac ligand was explored by DFT methods.
通过裂解六氟乙酰丙酮酸配体形成的 C 键三氟丙酮酸桥支持的钯二聚体的合成
众所周知,六氟乙酰丙酮酸钯(Pd(Hfacac)2)能与鲁替丁(2,6-二甲基吡啶)等碱形成加合物。Pd(Hfacac)2 与大约 3 等量的鲁替丁处理后,会产生文献中报道的 1:1 复合物 Pd(O,O-Hfacac)(C-Hfacac)(鲁替丁),即 1。然而,当过量鲁替丁的量增加时,会形成新的复合物 2。2 的单晶 X 射线结构证明,它是一种罕见的二聚钯配合物,含有两个 Pd(Hfacac)(卢替丁)片段,由二离子三氟丙酮酸配体 μ-CHC(O)CF3 桥接。2 的形成伴随着一种白色沉淀,经测定是反式-Pd(O2CCF3)2(lutidine)2 的混合物 (3),证实了 Hfac 配体裂解过程中缺失的三氟乙酸盐片段和 [lutidinium][Hfacac](4)的去向。随后的实验揭示了水在这一反应中的决定性作用。通过 DFT 方法探索了 Hfac 配体的裂解机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organometallics
化学-无机化学与核化学
CiteScore
5.60
自引率
7.10%
发文量
382
审稿时长
1.7 months
期刊介绍:
Organometallics is the flagship journal of organometallic chemistry and records progress in one of the most active fields of science, bridging organic and inorganic chemistry. The journal publishes Articles, Communications, Reviews, and Tutorials (instructional overviews) that depict research on the synthesis, structure, bonding, chemical reactivity, and reaction mechanisms for a variety of applications, including catalyst design and catalytic processes; main-group, transition-metal, and lanthanide and actinide metal chemistry; synthetic aspects of polymer science and materials science; and bioorganometallic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


