Mechanistic Studies on Rh(III)-Catalyzed Defluorinative Annulation of N-Sulfonylarylamides with Ethyl 2-Diazo-3,3,3-Trifluoropropanoates
IF 2.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The synthesis of fluorinated isoquinoline derivatives holds significant value in organic synthesis and medicine. Research into the reaction mechanisms and possible pathways for synthesizing these compounds plays a crucial role in advancing the development and applications of isoquinoline derivatives. Using density functional theory methods, we explored the reaction mechanism and potential pathways of the Rh(III)-catalyzed defluorinative annulation of N-sulfonylarylamides with ethyl 2-diazo-3,3,3-trifluoropropanoates. Theoretical calculations indicate that the reaction initiates with the formation of a metal carbene via C–H activation and denitrogenation, followed by migratory insertion. Subsequent steps involve metal-assisted β-fluoride elimination and anion exchange. Finally, intramolecular cyclization, defluorination, and sulfonyl migration yield isoquinoline products.
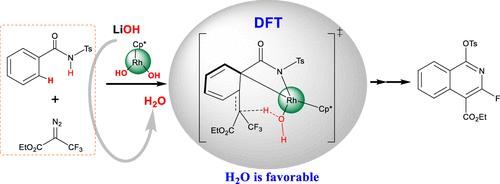
Rh(III)-Catalyzed Defluorinative Annulation of N-Sulfonylarylamides with Ethyl 2-Diazo-3,3,3-Trifluoropropanoates 的机理研究
含氟异喹啉衍生物的合成在有机合成和医药领域具有重要价值。研究合成这些化合物的反应机理和可能途径对促进异喹啉衍生物的开发和应用起着至关重要的作用。我们利用密度泛函理论方法,探索了 Rh(III) 催化的 N-磺酰基芳酰胺与 2-重氮-3,3,3-三氟丙酸乙酯的脱氟环化反应机理和潜在途径。理论计算表明,该反应首先通过 C-H 活化和脱氮形成金属碳烯,然后进行迁移插入。随后的步骤包括金属辅助的 β-氟消除和阴离子交换。最后,分子内环化、脱氟和磺酰迁移产生异喹啉产物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organometallics
化学-无机化学与核化学
CiteScore
5.60
自引率
7.10%
发文量
382
审稿时长
1.7 months
期刊介绍:
Organometallics is the flagship journal of organometallic chemistry and records progress in one of the most active fields of science, bridging organic and inorganic chemistry. The journal publishes Articles, Communications, Reviews, and Tutorials (instructional overviews) that depict research on the synthesis, structure, bonding, chemical reactivity, and reaction mechanisms for a variety of applications, including catalyst design and catalytic processes; main-group, transition-metal, and lanthanide and actinide metal chemistry; synthetic aspects of polymer science and materials science; and bioorganometallic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


