Electrocatalytic Anaerobic Oxidation of Benzylic Amines Enabled by Ferrocene-Based Redox Mediators
IF 2.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The generation and functionalization of carbon- or nitrogen-centered radicals are of great interest for their potential synthetic utility. Here, we report the anaerobic electrocatalytic oxidation of two primary benzylic amines, benzylamine and 2-picolylamine, in the presence of a catalytic quantity of an electron deficient ferrocene derivative as a single-electron redox mediator. The use of the appropriate redox mediator prevented fouling of the electrode surface and significantly decreased the potential at which the catalytic oxidation reaction occurred. Simulation of the electrochemical results revealed an ErCi′ catalytic process between the redox mediator and both substrates and significant difference in the electron transfer rate between the two substrates and electrochemically oxidized mediator. Through anaerobic controlled-potential electrolysis, we demonstrated a method with a Faradaic efficiency of 90% forming the desired coupled imine product of benzylamine oxidation while avoiding an excess of problematic overoxidation, hydrolysis, and other side reactions. Based on the electrochemical data along with the product analyses using IR and 1H and 13C NMR spectroscopies, the proposed mechanistic steps for the redox mediated electrocatalytic process were laid out.
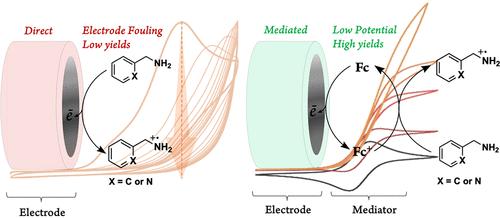
二茂铁基氧化还原介质促成的苄胺电催化厌氧氧化作用
碳或氮中心自由基的生成和功能化因其潜在的合成用途而备受关注。在此,我们报告了在催化量的二茂铁衍生物作为单电子氧化还原介质存在的情况下,两种伯氨苄基胺(苄胺)和 2-匹考胺(2-picolylamine)的厌氧电催化氧化过程。使用适当的氧化还原介质可以防止电极表面结垢,并显著降低催化氧化反应发生时的电位。对电化学结果的模拟显示,氧化还原介质和两种底物之间存在 ErCi′ 催化过程,两种底物和电化学氧化介质之间的电子转移率存在显著差异。通过厌氧控制电位电解,我们展示了一种法拉第效率高达 90% 的方法,它能在苄胺氧化过程中形成所需的偶联亚胺产物,同时避免了过量的过氧化、水解和其他副反应等问题。根据电化学数据以及利用红外光谱、1H 和 13C NMR 光谱进行的产物分析,我们提出了氧化还原介导电催化过程的机械步骤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organometallics
化学-无机化学与核化学
CiteScore
5.60
自引率
7.10%
发文量
382
审稿时长
1.7 months
期刊介绍:
Organometallics is the flagship journal of organometallic chemistry and records progress in one of the most active fields of science, bridging organic and inorganic chemistry. The journal publishes Articles, Communications, Reviews, and Tutorials (instructional overviews) that depict research on the synthesis, structure, bonding, chemical reactivity, and reaction mechanisms for a variety of applications, including catalyst design and catalytic processes; main-group, transition-metal, and lanthanide and actinide metal chemistry; synthetic aspects of polymer science and materials science; and bioorganometallic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


