Metal oxyhalide-based heterogeneous catalytic water purification with ultralow H2O2 consumption
引用次数: 0
Abstract
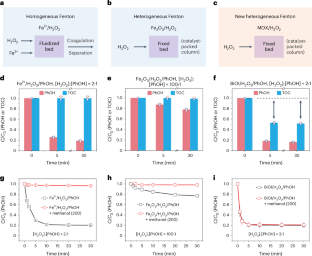
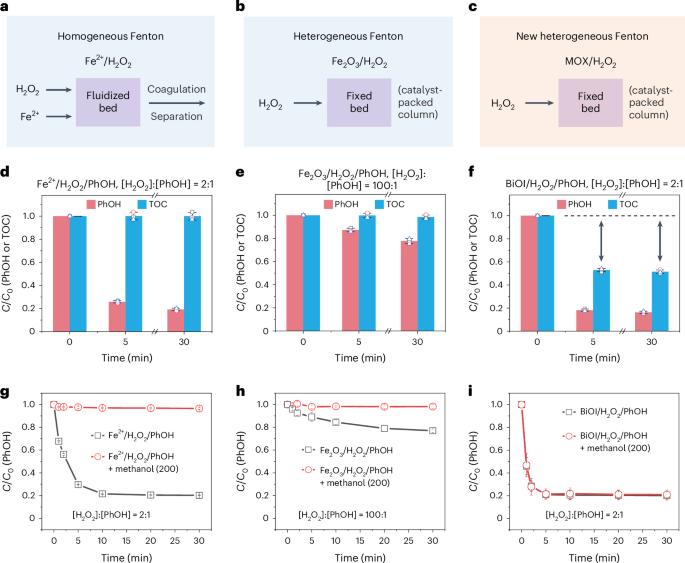
In the quest for advanced water treatment via Fenton and Fenton-like reactions, minimizing the hydrogen peroxide (H2O2) usage by improving its activation efficiency is a critical goal. Here we report a metal oxyhalide (MOX)-based Fenton reaction system that differs fundamentally from traditional ones in pollutant removal pathway and mechanism. The MOX/H2O2 system enables efficient coupling and polymerization of organic pollutants via mild surface direct oxidation, bypassing the generation of reactive oxygen species. As a result, pollutants are translocated and removed from water with ultralow H2O2 consumption, avoiding the formation of toxic by-products. It achieves up to 80% pollutant (50% total organic carbon) removal at a H2O2-to-pollutants molar ratio of 2:1, outperforming conventional Fenton systems, which are operated at ratios ranging from 20:1 to 1,000:1. The success of these catalytic systems is attributed to the synergistic actions of O-bridging M and X sites on the catalyst surface, which selectively activate pollutants and H2O2, respectively. The catalyst could be extended to low-cost and environmentally benign MOX materials such as BiOI, FeOCl and VOCl, and be adopted to construct a dynamic membrane filtration catalytic system for high-performance and energy-saving abatement of micropollutants in water, providing a promising water purification paradigm. Traditional Fenton and Fenton-like reactions for pollutant removal require a substantial amount of H2O2. In contrast, the heterogeneous metal oxyhalide-based Fenton catalytic approach achieves organic pollutant removal by concentrating and activating them on the catalyst, significantly minimizing H2O2 consumption.
超低 H2O2 消耗量的金属氧卤化物基异相催化水净化技术
在寻求通过芬顿和类芬顿反应进行先进水处理的过程中,通过提高过氧化氢(H2O2)的活化效率最大限度地减少其用量是一个关键目标。在此,我们报告了一种基于金属氧卤化物(MOX)的芬顿反应系统,该系统在污染物去除途径和机理方面与传统的芬顿反应系统有着本质区别。MOX/H2O2 系统通过温和的表面直接氧化作用,绕过活性氧的生成,实现了有机污染物的高效耦合和聚合。因此,污染物从水中转移和清除的 H2O2 消耗量极低,避免了有毒副产品的形成。当 H2O2 与污染物的摩尔比为 2:1 时,它的污染物(50% 总有机碳)去除率高达 80%,优于传统的 Fenton 系统,后者的比率从 20:1 到 1,000:1 不等。这些催化系统的成功归功于催化剂表面的 O 桥 M 位点和 X 位点的协同作用,它们分别选择性地激活污染物和 H2O2。该催化剂可推广到 BiOI、FeOCl 和 VOCl 等低成本、对环境无害的 MOX 材料上,并可用于构建动态膜过滤催化系统,以高性能、节能地去除水中的微污染物,提供一种前景广阔的水净化范例。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


