Investigations on electrical, electrochemical, and thermal properties of gelatine-based novel biopolymer electrolytes for energy storage applications
Abstract
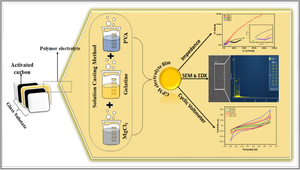
Economical and environmentally friendly natural polymer electrolytes are prepared using a combination of gelatine, polyvinyl alcohol (PVA), and magnesium chloride (MgCl2) by solution casting technique. These electrolytes exhibit enhanced amorphous properties confirmed by X-ray diffraction (XRD). The homogeneous surface is confirmed by scanning electron microscopy (SEM), and the elemental composition is confirmed by energy-dispersive X-ray (EDX) spectroscopy. The thermal stability of the biopolymer electrolytes is determined between 0 and 350 °C by thermal gravimetry analysis (TGA). Electrochemical impedance spectroscopy (EIS) confirms the higher ionic conductivity of 4.1 × 10−3 S/cm for Gelatine:PVA:MgCl2 (0.5 g:0.5 g:0.4 g) electrolyte at normal temperature. The cyclic voltammetry (CV) curve reveals the non-faradaic behavior of electrolytes. The specific capacitance records 23.9 F/g at 10 mV/s scan rate and drops to 7.1 F/g at 150 mV/s, a higher scan rate. For the higher conducting material, linear sweep voltammetry (LSV) analysis reveals a potential window of − 1.2 to + 1 V. According to the research, this solid polymer electrolyte may be a good choice for electrochemical devices because of their excellent electrochemical behavior. The objective of this research is to explore the potential of these solid polymer electrolytes for use in electrochemical devices, given their excellent electrochemical behavior. Future work will focus on optimizing the electrolyte composition and further enhancing their electrochemical performance for practical applications in energy storage devices.
Graphical Abstract

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


