A Facile Synthesis Strategy of Supported g-C3N4 and Molybdenum Disulfide over Co3O4 Spinal Composite Nanostructure: An Excellent Catalyst for HER
IF 5.3
3区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
Abstract
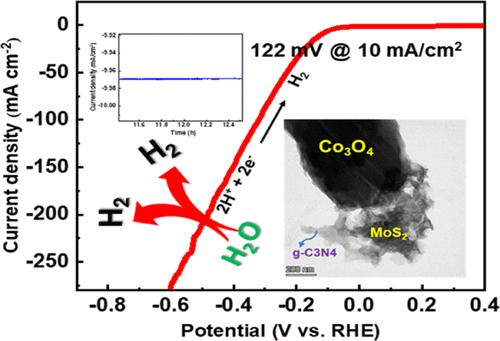
To ensure the sustainability of energy, dihydrogen (H2) constitutes the best alternative to fossil fuels for a fully renewable and clean energy carrier with the highest energy density. The effective and abundant electrocatalysis of water is crucial for achieving large-scale practical hydrogen evolution. This study introduces a simple and efficient method for synthesizing a Co3O4/MoS2@g-C3N4 (CMCN) heterostructure catalyst. The CMCN composite material has effective hydrogen evolution reaction (HER) kinetic activity across several experimental configurations, indicating its desirability. The CMCN composite demonstrates exceptional performance in the hydrogen evolution reaction (HER) in 0.5 M H2SO4, with a minimal overpotential of 122 mV at a current density of −10 mA cm-2 and a narrow Tafel slope of 49 mVdec-1, surpassing pristine Co3O4, MoS2, g-C3N4, and Co3O4/MoS2 (CM) composite. The heterostructure design promotes efficient charge transfer and optimizes the hydrogen adsorption/desorption processes. Interestingly, composite catalysts’ high current density and high mass activity ensure exceptional catalyst performance, along with low overpotential, minimal charge transfer, and a low Tafel value. This adaptable technique also makes it possible to design and create previously unheard-of inexpensive mixed metal electrocatalysts.
Co3O4脊柱复合纳米结构上支撑的 g-C3N4 和二硫化钼的简便合成策略:一种优异的 HER 催化剂
为确保能源的可持续性,二氢(H2)是化石燃料的最佳替代品,它是一种完全可再生的清洁能源载体,具有最高的能量密度。有效而丰富的水电催化是实现大规模实用氢进化的关键。本研究介绍了一种简单高效的 Co3O4/MoS2@g-C3N4 (CMCN) 异质结构催化剂的合成方法。CMCN 复合材料在几种实验配置中都具有有效的氢气进化反应(HER)动力学活性,表明其具有可取性。CMCN 复合材料在 0.5 M H2SO4 中的氢进化反应(HER)中表现出卓越的性能,在电流密度为 -10 mA cm-2 时,过电位最低为 122 mV,塔菲尔斜率为 49 mVdec-1,超过了原始 Co3O4、MoS2、g-C3N4 和 Co3O4/MoS2 (CM) 复合材料。异质结构设计促进了高效电荷转移,优化了氢吸附/解吸过程。有趣的是,复合催化剂的高电流密度和高质量活性确保了催化剂的卓越性能,同时还具有低过电位、最小电荷转移和低塔菲尔值等特点。这种适应性强的技术还使得设计和制造以前闻所未闻的廉价混合金属电催化剂成为可能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy & Fuels
工程技术-工程:化工
CiteScore
9.20
自引率
13.20%
发文量
1101
审稿时长
2.1 months
期刊介绍:
Energy & Fuels publishes reports of research in the technical area defined by the intersection of the disciplines of chemistry and chemical engineering and the application domain of non-nuclear energy and fuels. This includes research directed at the formation of, exploration for, and production of fossil fuels and biomass; the properties and structure or molecular composition of both raw fuels and refined products; the chemistry involved in the processing and utilization of fuels; fuel cells and their applications; and the analytical and instrumental techniques used in investigations of the foregoing areas.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


