In-situ fabrication of BiOCl/OVs–BiPO4 heterojunctions with enhanced photocatalytic destruction performance
Abstract
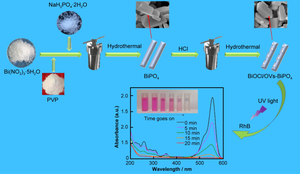
In this work, BiOCl/OVs–BiPO4 heterojunction photocatalysts were successfully in-situ prepared by treating of BiPO4 with dilute hydrochloric acid (HCl) under hydrothermal condition. Systematically characterization results confirm that BiOCl/BiPO4 heterojunctions have been successfully in-situ constructed and oxygen vacancies (OVs) are significantly increased. The OVs on the surface of the BiOCl/OVS–BiPO4 heterojunctions photocatalyst and the interface electric field at the interface of the heterojunctions effectively accelerate the separation and migration of photogenerated carriers, and the surface OVs provide more sites for adsorption and reaction. Consequently, BiOCl/OVs–BiPO4 heterojunction photocatalysts have higher separation rate of photoexcited e−/h+ pairs and exhibit ascendant photocatalytic degradation activity. Electron paramagnetic resonance (EPR) technology and free radical capture experiments give strong evidence that ·O2− exists in the reaction system and is the leading species during the degradation process. The experimental results reveal that the degradation efficiency of rhodamine B (RhB) over BiPO4 treated with 3 ml of 0.1% dilute hydrochloric acid (3HCl-BPO) is 2.42 times of that over the reference BiPO4. After ultraviolet (UV) light illumination for 20 min, the destruction degree of RhB on the 3HCl-BPO sample reaches 99%. Moreover, the degradation rate of tetracycline (TC) is also obviously improved over 3HCl-BPO compared with that on the reference BiPO4 after 40 min exposure to ultraviolet light. The excellent stability of the sample was demonstrated by five cycles. A reasonable enhancement mechanism for BiOCl/OVs–BiPO4 heterojunctions was proposed to elucidate the boosted photocatalytic performance. This work offers a facile and reliable reference to design high performance BiPO4-based photocatalysts for environment purification.
Graphical abstract

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


