Oxidation of diols using a substoichiometric quantity of an oxoammonium salt bearing the nitrate anion
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The oxidation of diols to lactones, dialdehydes and diketones using a sub-stoichiometric quantity of an oxoammonium salt bearing the nitrate counterion is reported. In the case of linear aliphatic diols, when the diol substrate is shorter-chained, the lactone product predominates. When the chain is longer, the dialdehyde is the major product formed. A number of cyclic diols are also converted to their lactone, diketone or hydroxyketone congeners.
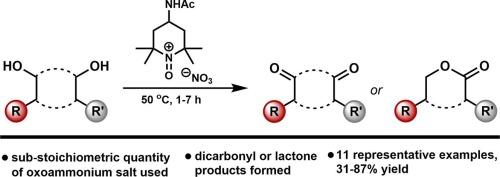
使用亚几何量的含硝酸阴离子的氧化铵盐氧化二元醇
报告中介绍了使用亚几何量的含硝酸反离子的氧化铵盐将二元醇氧化成内酯、二醛和二酮的过程。就线性脂肪族二元醇而言,当二元醇底物的链较短时,内酯产物占主导地位。当链较长时,形成的主要产物是二醛。一些环状二元醇也会转化为内酯、二酮或羟基酮同系物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


