Brønsted acid catalyzed N2‑selective alkylation of 1,2,3-triazoles with trichloroacetimidates
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A Brønsted acid catalyzed alkylation method for 1,2,3-triazoles is described using trichloroacetimidates as the electrophiles. These conditions were selective, with a strong preference of N2 alkylation product, often in high yield. The ratio of N2:N1 alkylation is sensitive to both the type of solvent used and the reaction concentration. The optimal results were obtained with a non-polar solvent at higher dilutions. Both 1,2,3-triazoles and 1,2,3-benzotriazoles could be alkylated under these conditions, providing access to N2 substituted 1,2,3-triazoles in good yields.
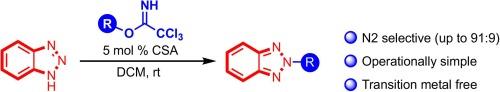
布氏酸催化的 1,2,3-三唑与三氯乙酰亚氨酸盐的 N2 选择性烷基化反应
介绍了一种以三氯乙酰亚胺酸盐为亲电体的 1,2,3-三唑的布氏酸催化烷基化方法。这些条件具有选择性,N2 烷基化产物具有很强的偏好性,通常产量很高。N2:N1 烷基化的比例对所用溶剂的类型和反应浓度都很敏感。在较高的稀释度下,使用非极性溶剂可获得最佳结果。在这些条件下,1,2,3-三唑和 1,2,3-苯并三唑都能被烷基化,从而以良好的收率获得 N2 取代的 1,2,3-三唑。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


