Electrochemical Reduction of Nitrogen to Ammonia Using Zinc Telluride
IF 5.7
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
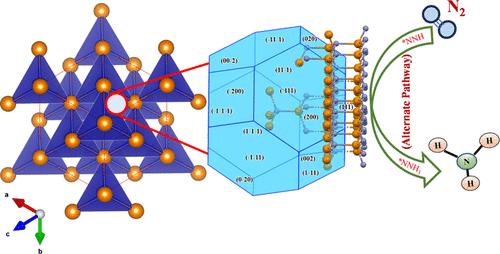
Electrosynthesis of ammonia (NH3), an important constituent molecule of various commercial fertilizers, is a promising and sustainable alternative strategy compared with the century-old Haber-Bosch process. Herein, zinc telluride (ZnTe) is demonstrated as an efficient electrocatalyst for reducing nitrogen (N2) under ambient conditions to NH3. In this simple chemical strategy, Zn preferentially binds N2 over hydrogen (H2), and Te, by virtue of its superior electronic properties, enhances the electrocatalytic activity of ZnTe. The analysis of the X-ray diffraction data using the Bravais-Friedel-Donnay-Harker (BFDH) theory predicted a crystal geometry with the active electrocatalytic sites predominantly confined to the (111) planes of ZnTe. The preferential binding of nitrogen (N2; adsorption energy = −0.043 eV) over hydrogen (H2, adsorption energy = −0.028 eV) to Zn on the (111) plane of ZnTe is further confirmed by density functional theory. The ZnTe catalyst is observed to be stable in the acidic medium and delivers a very high yield of NH3 (19.85 μg/h–1 mgcat–1) and a Faradaic efficiency of 6.24% at −0.6 V (versus RHE). Additional verification experiments do not reveal the formation of side products (such as NH2–NH2) during N2 reduction by ZnTe. Further, density functional theory calculations strongly predict that the electrocatalytic reduction of N2 to NH3 by ZnTe preferentially occurs via the alternate pathway.
利用碲化锌电化学还原氮气至氨气
氨(NH3)是各种商业化肥的重要组成分子,与具有百年历史的哈伯-博施工艺相比,电合成氨是一种前景广阔的可持续替代策略。在本文中,碲化镉锌(ZnTe)被证明是一种高效的电催化剂,可在环境条件下将氮(N2)还原为 NH3。在这一简单的化学策略中,锌优先结合 N2 而不是氢 (H2),而 Te 则凭借其优异的电子特性增强了 ZnTe 的电催化活性。利用布拉维-弗里德尔-多奈-哈克(BFDH)理论对 X 射线衍射数据进行分析后,预测了 ZnTe 的晶体几何结构,其活性电催化位点主要集中在 (111) 平面上。密度泛函理论进一步证实,氮(N2;吸附能 = -0.043 eV)比氢(H2,吸附能 = -0.028 eV)优先与 ZnTe (111) 平面上的 Zn 结合。据观察,ZnTe 催化剂在酸性介质中非常稳定,并能提供非常高的 NH3 产量(19.85 μg/h-1 mgcat-1),在 -0.6 V 电压下的法拉第效率为 6.24%(相对于 RHE)。额外的验证实验没有发现 ZnTe 在还原 N2 的过程中形成副产物(如 NH2-NH2)。此外,密度泛函理论计算还有力地预测了 ZnTe 通过电催化将 N2 还原成 NH3 的过程主要是通过交替途径进行的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Materials Au
材料科学-
CiteScore
5.00
自引率
0.00%
发文量
0
期刊介绍:
ACS Materials Au is an open access journal publishing letters articles reviews and perspectives describing high-quality research at the forefront of fundamental and applied research and at the interface between materials and other disciplines such as chemistry engineering and biology. Papers that showcase multidisciplinary and innovative materials research addressing global challenges are especially welcome. Areas of interest include but are not limited to:Design synthesis characterization and evaluation of forefront and emerging materialsUnderstanding structure property performance relationships and their underlying mechanismsDevelopment of materials for energy environmental biomedical electronic and catalytic applications
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


