Synthetic Transformations of Higher Terpenoids. 45#. Regioselective Synthesis of 5-Labdanoid-Substituted Pyrazoles and Assessment of Their Analgesic Activity
IF 0.8
4区 化学
Q4 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
A method for targeted structural modification of the methyl ester of phlomisoic acid was developed by introduction of an additional C-16 ketopyrazole moiety via cyclocondensation of diterpenoid alkyne-1,2- diones with arylhydrazines. The conditions for regioselective formation of labdanoid 1,3-diarylpyrazol-5- ylmethanones were found. The possibility of a sequential four-component reaction in a single pot was demonstrated. Significant analgesic activity of labdanoid-substituted diarylpyrazoles in thermal and chemical irritation models was established.
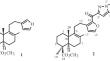

高等萜类化合物的合成转化。45#.5-Labdanoid-Substituted Pyrazoles 的区域选择性合成及其镇痛活性评估
通过二萜炔烃-1,2-二酮与芳基肼的环缩合作用,引入额外的 C-16 酮吡唑分子,开发了一种对 phlomisoic 酸甲酯进行定向结构修饰的方法。找到了区域选择性形成 1,3-二芳基吡唑-5-甲酮的条件。证明了在一个反应釜中连续进行四组份反应的可能性。在热刺激和化学刺激模型中,证实了拉巴旦类取代的二芳基吡唑具有显著的镇痛活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Natural Compounds
化学-有机化学
CiteScore
1.40
自引率
25.00%
发文量
265
审稿时长
7.8 months
期刊介绍:
Chemistry of Natural Compounds publishes reviews and general articles about the structure of different classes of natural compounds, the chemical characteristics of botanical families, genus, and species, to establish the comparative laws and connection between physiological activity and the structure of substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


