Prominent Role of Charge Transfer in the Spectral Tuning of Photosynthetic Light-Harvesting I Complex
IF 3.7
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
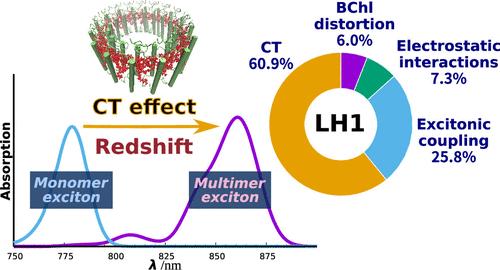
Purple bacteria possess two ring-shaped protein complexes, light-harvesting 1 (LH1) and 2 (LH2), both of which function as antennas for solar energy utilization for photosynthesis but exhibit distinct absorption properties. The two antennas have differing amounts of bacteriochlorophyll (BChl) a; however, their significance in spectral tuning remains elusive. Here, we report a high-precision evaluation of the physicochemical factors contributing to the variation in absorption maxima between LH1 and LH2, namely, BChl a structural distortion, protein electrostatic interaction, excitonic coupling, and charge transfer (CT) effects, as derived from detailed spectral calculations using an extended version of the exciton model, in the model purple bacterium Rhodospirillum rubrum. Spectral analysis confirmed that the electronic structure of the excited state in LH1 extended to the BChl a 16-mer. Further analysis revealed that the LH1-specific redshift (∼61% in energy) is predominantly accounted for by the CT effect resulting from the closer inter-BChl distance in LH1 than in LH2. Our analysis explains how LH1 and LH2, both with chemically identical BChl a chromophores, use distinct physicochemical effects to achieve a progressive redshift from LH2 to LH1, ensuring efficient energy transfer to the reaction center special pair.
电荷转移在光合作用光收集 I 复合物光谱调谐中的突出作用
紫色细菌拥有两种环形蛋白质复合物,即采光 1(LH1)和采光 2(LH2),它们都是利用太阳能进行光合作用的天线,但具有不同的吸收特性。这两个天线具有不同数量的细菌叶绿素(BChl)a;然而,它们在光谱调谐中的意义仍然难以捉摸。在此,我们报告了对导致 LH1 和 LH2 之间吸收最大值变化的物理化学因素(即 BChl a 结构畸变、蛋白质静电相互作用、激子耦合和电荷转移(CT)效应)的高精度评估,这些因素是通过使用扩展版激子模型对紫色红球菌(Rhodospirillum rubrum)进行详细光谱计算得出的。光谱分析证实,LH1 激发态的电子结构延伸至 BChl a 16-mer。进一步的分析表明,LH1 特定的红移(能量的 61%)主要是由 LH1 中比 LH2 中更近的 BChl 间距所产生的 CT 效应引起的。我们的分析解释了化学性质完全相同的 BChl a 发色团 LH1 和 LH2 如何利用不同的物理化学效应实现从 LH2 到 LH1 的渐进红移,从而确保向反应中心特殊配对进行有效的能量转移。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.70
自引率
0.00%
发文量
0
期刊介绍:
ACS Physical Chemistry Au is an open access journal which publishes original fundamental and applied research on all aspects of physical chemistry. The journal publishes new and original experimental computational and theoretical research of interest to physical chemists biophysical chemists chemical physicists physicists material scientists and engineers. An essential criterion for acceptance is that the manuscript provides new physical insight or develops new tools and methods of general interest. Some major topical areas include:Molecules Clusters and Aerosols; Biophysics Biomaterials Liquids and Soft Matter; Energy Materials and Catalysis

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


