Sculpting the tunable mesoscopic helical chirality into poly(m-phenylenediamine) via Mn2+ coordination
Abstract
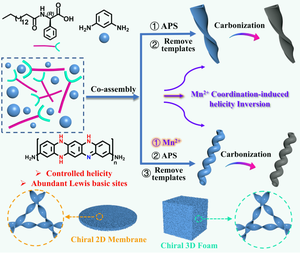
Chiral conjugated polymers with controlled mesoscopic helicity are gaining attention for enantioseparation and asymmetric catalysis. However, achieving on-demand chirality and processability remain challenging. Herein, we exploit supramolecular coordination polymers formed by Mn2+ and chiral phenylglycine derivatives (L-/D-16PhgCOOH) as templates, using m-phenylenediamine as the monomer to synthesize chiral poly(m-phenylenediamine) (PMPD). In the Mn2+-templated system, the PMPD’s handedness is opposite to the molecular chirality of L-/D-16PhgCOOH, while in the Mn2+-free system, the PMPD handedness aligns with that of the template molecule. This method allows for helicity switching of chiral polymers within a single chirality template system. The introduction of Mn2+ is demonstrated to disrupt and reconstitute the supramolecular interactions in the co-assembly, influencing subsequent supramolecular stacking patterns. Carbonizing the resulting PMPDs directly produces chiroptical active nitrogen-doped carbonaceous nanomaterials that inherit the original helicity. Moreover, incorporating F-127 into the polymerization system enhances the aspect ratio of PMPDs, facilitating their delicate processing into chiral self-supporting two-dimensional films and three-dimensional foams. With abundant Lewis basic sites, these chiral polymers offer versatile platforms for novel chiral host-guest interactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


