Divalent vinyl ketones derived from fluorene: a facile synthesis of bifunctional acrylic monomers with high reactivity in thia-/aza-Michael addition and Morita-Baylis-Hillman reactions
IF 2.3
4区 化学
Q3 POLYMER SCIENCE
引用次数: 0
Abstract
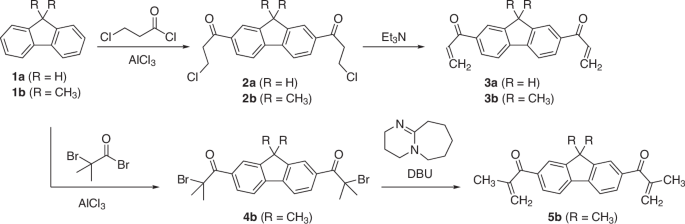
Although vinyl ketones (VKs) exhibit excellent reactivity toward radicals and nucleophiles, their application in polymer chemistry has been limited compared with that of acrylates. One of the reasons is the difficulty of the synthesis, particularly that of multivalent VKs. Herein, we report the facile synthesis of divalent VKs via Friedel‒Crafts acylation of fluorene and subsequent elimination reactions. For example, via this approach, 2,7-diacryloyl-9,9-dimethylfluorene was obtained at high yields (78%). Because the monomer was obtained at high purity through recrystallization and washing, the procedure is suitable for industrial applications. The addition of dithiols and diamines via thia- and aza-Michael addition afforded the corresponding polythioesters and polyamines, respectively. In addition, the divalent VKs exhibited high reactivity in the Morita‒Baylis‒Hillman reaction with formaldehyde, affording a diol monomer. The polycondensation of the diol monomer and isophthaloyl dichloride yielded a poly(conjugated-ketone ester). Consequently, the divalent VKs described herein are attractive monomers and monomer precursors with ready accessibility and sufficient electrophilicity. A series of divalent vinyl ketones containing fluorene backbone were synthesized via Friedel-Crafts acylation and subsequent elimination reactions. The divalent vinyl ketones underwent polyaddition with dithiols to yield the corresponding polysulfide via thiol–ene click chemistry. They also exhibited high reactivity in the Baylis-Hillman reaction with formaldehyde to afford a diol monomer, and the polycondensation with isophthaloyl dichloride yielded a poly(conjugated-ketone ester).

源自芴的二价乙烯酮:在噻-/扎-迈克尔加成反应和莫里塔-贝利斯-希尔曼反应中具有高反应活性的双官能丙烯酸单体的简便合成方法
尽管乙烯基酮(VKs)对自由基和亲核试剂表现出优异的反应性,但与丙烯酸酯相比,它们在聚合物化学中的应用受到限制。其中一个原因是合成困难,特别是多价VKs的合成困难。在这里,我们报道了通过氟的Friedel-Crafts酰化和随后的消除反应,容易合成二价VKs。例如,通过这种方法,2,7-二丙烯酰-9,9-二甲基芴的产率很高(78%)。由于该方法通过再结晶和洗涤获得了高纯度的单体,因此适合工业应用。二硫醇和二胺分别通过thia-和aza-Michael加成得到相应的聚硫酯和多胺。此外,二价VKs在与甲醛的Morita-Baylis-Hillman反应中表现出较高的反应活性,生成了二醇单体。二醇单体与二氯异苯二甲酰缩聚制得聚共轭酮酯。因此,本文所述的二价vk是有吸引力的单体和单体前体,具有现成的可及性和足够的亲电性。通过Friedel-Crafts酰化和消除反应合成了一系列含芴骨架的二价乙烯基酮。二价乙烯基酮与二硫醇通过巯基键反应得到相应的多硫化物。它们在与甲醛的Baylis-Hillman反应中也表现出很高的反应活性,得到二醇单体,与二氯异苯二甲酰缩聚得到聚(共轭酮酯)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Journal
化学-高分子科学
CiteScore
5.60
自引率
7.10%
发文量
131
审稿时长
2.5 months
期刊介绍:
Polymer Journal promotes research from all aspects of polymer science from anywhere in the world and aims to provide an integrated platform for scientific communication that assists the advancement of polymer science and related fields. The journal publishes Original Articles, Notes, Short Communications and Reviews.
Subject areas and topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Polymer synthesis and reactions
Polymer structures
Physical properties of polymers
Polymer surface and interfaces
Functional polymers
Supramolecular polymers
Self-assembled materials
Biopolymers and bio-related polymer materials
Polymer engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


