Structural basis of LRPPRC–SLIRP-dependent translation by the mitoribosome
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
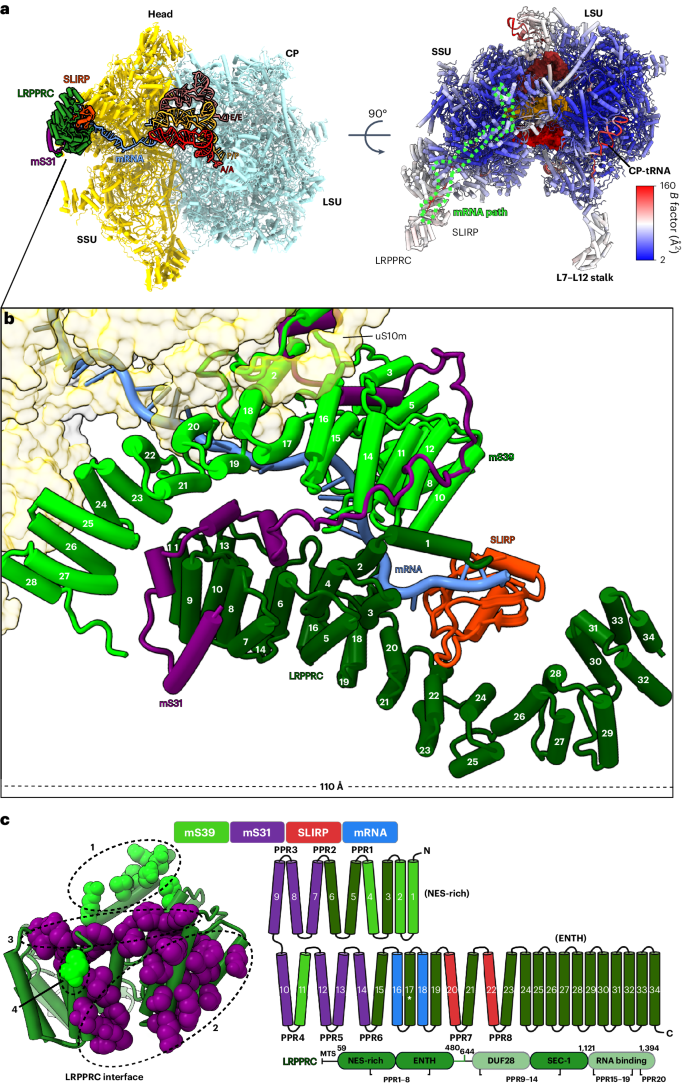
In mammalian mitochondria, mRNAs are cotranscriptionally stabilized by the protein factor LRPPRC (leucine-rich pentatricopeptide repeat-containing protein). Here, we characterize LRPPRC as an mRNA delivery factor and report its cryo-electron microscopy structure in complex with SLIRP (SRA stem-loop-interacting RNA-binding protein), mRNA and the mitoribosome. The structure shows that LRPPRC associates with the mitoribosomal proteins mS39 and the N terminus of mS31 through recognition of the LRPPRC helical repeats. Together, the proteins form a corridor for handoff of the mRNA. The mRNA is directly bound to SLIRP, which also has a stabilizing function for LRPPRC. To delineate the effect of LRPPRC on individual mitochondrial transcripts, we used RNA sequencing, metabolic labeling and mitoribosome profiling, which showed a transcript-specific influence on mRNA translation efficiency, with cytochrome c oxidase subunit 1 and 2 translation being the most affected. Our data suggest that LRPPRC–SLIRP acts in recruitment of mitochondrial mRNAs to modulate their translation. Collectively, the data define LRPPRC–SLIRP as a regulator of the mitochondrial gene expression system. Here, using cryo-electron microscopy to study the structure of LRPPRC (leucine-rich pentatricopeptide repeat-containing protein) in complex with SLIRP (SRA stem-loop-interacting RNA-binding protein), mRNA and the mitoribosome, the authors show that LRPPRC facilitates mRNA handoff to the mitoribosome and regulates the expression of several mitochondrial genes.

米托里伯体依赖 LRPPRC-SLIRP 翻译的结构基础
在哺乳动物线粒体中,mRNA 通过蛋白因子 LRPPRC(含亮氨酸丰富五肽重复蛋白)同转录稳定。在这里,我们描述了 LRPPRC 作为 mRNA 递送因子的特性,并报告了它与 SLIRP(SRA 干环相互作用 RNA 结合蛋白)、mRNA 和 mitoribosome 复合物的冷冻电镜结构。该结构显示,LRPPRC通过识别LRPPRC螺旋重复序列与mitoribosomal蛋白mS39和mS31的N末端结合。这些蛋白共同形成了一个 mRNA 的交接通道。mRNA 直接与 SLIRP 结合,而 SLIRP 对 LRPPRC 也有稳定作用。为了明确 LRPPRC 对线粒体单个转录本的影响,我们使用了 RNA 测序、代谢标记和 mitoribosome 分析方法,结果显示转录本对 mRNA 翻译效率的影响具有特异性,其中环氧化酶 1 和 2 的翻译受到的影响最大。我们的数据表明,LRPPRC-SLIRP 在线粒体 mRNA 的招募过程中起着调节其翻译的作用。总之,这些数据确定了 LRPPRC-SLIRP 是线粒体基因表达系统的调控因子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


