Chiral stacks of a curved nanographene
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Despite enormous advances in the edge extension chemistry of nanographenes, examples of peri-annulations and the knowledge of their effect on molecular properties remain scarce. Here, we show the synthesis of a curved C60S5 nanographene comprising quintuple [5]thiahelicenes arranged in a C5-symmetric fashion on the zigzag edge (L-region) of a bowl-shaped corannulene core. The synthesis is achieved with the help of Stille coupling, alkynyl thiolation, sulfide/aryne cyclization, and direct arylation reactions. The prepared bowl-helix chiral structure absorbs and emits in the visible and near-IR regions. It assembles into persistent molecular bilayer graphene stacks in solution, solid state, and gas phase. The concave cavities of the supramolecular dimers can recognize the convex surfaces of fullerene C60 through shape complementarity and π-π stacking interactions in the solid state. A properties comparison with ortho-annulated analogs and archetypical nanographenes indicates the superiority of peri-annulations in the design of molecular graphenes.
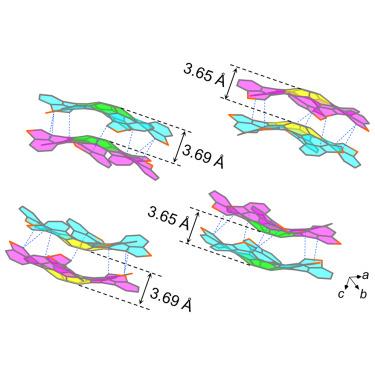
弯曲纳米石墨的手性叠层
尽管在纳米石墨烯的边缘延伸化学方面取得了巨大进步,但围嵌合的实例及其对分子特性影响的知识仍然很少。在这里,我们展示了一种弯曲的 C60S5 纳米石墨烯的合成方法,它由五倍 [5]thiahelicenes 组成,以 C5 对称的方式排列在碗形茱萸核的人字形边缘(L 区域)上。该合成是通过斯蒂尔偶联、炔基硫代、硫化物/芳基环化和直接芳基化反应实现的。所制备的碗状螺旋手性结构在可见光和近红外区域吸收和发光。它能在溶液、固态和气相中组装成持久的分子双层石墨烯堆栈。在固态下,超分子二聚体的凹腔可以通过形状互补和π-π堆叠相互作用识别富勒烯 C60 的凸面。与正嵌段类似物和原型纳米石墨烯的性质比较表明,围嵌段在设计分子石墨烯方面具有优越性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


