Quantitative 99mTc-pyrophosphate myocardial uptake: Changes on transthyretin stabilization therapy
IF 3
4区 医学
Q2 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background
Quantitative technetium-99m-pyrophosphate cardiac single-photon emission computed tomography (99mTc-PYP SPECT/CT) is an emerging method for estimating myocardial burden of transthyretin cardiac amyloidosis (ATTR-CA), but its efficacy in monitoring longitudinal changes remains uncertain. We aimed to investigate longitudinal changes in cardiac ATTR amyloid burden following transthyretin stabilization therapy using visual and quantitative 99mTc-PYP SPECT/CT and to relate these with changes in cardiac biomarkers and function.
Methods
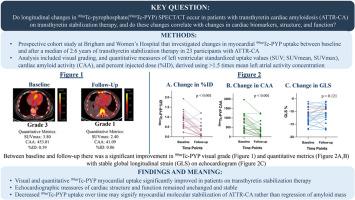
This prospective longitudinal cohort study investigated changes in 99mTc-PYP SPECT/CT in 23 participants with ATTR-CA on transthyretin stabilization therapy (median: 2.6 years). Quantitative analysis included left ventricular (LV) standardized uptake values (SUVs) (SUVmax, SUVmean), cardiac amyloid activity (CAA; SUVmean∗LV activity volume), and percent injected dose (%ID) (mean activity concentration∗LV activity volume/injected activity), calculated using a threshold of >1.5 times left atrial blood pool activity concentration on SPECT/CT. Longitudinal changes of paired continuous and ordinal variables were analyzed using Wilcoxon signed-rank test.
Results
Following therapy, visual grade decreased significantly (P = 0.003). Several quantitative 99mTc-PYP metrics also decreased significantly: SUVmax (median −0.75, P = 0.011), CAA (median: −406.6, P < 0.001), and %ID (median: −0.45, P < 0.001). Serum transthyretin levels improved (median: +6.5 mg/dL, P = 0.008). Echocardiographic parameters (global longitudinal strain, LV mass index, and LV wall thickness), N-terminal pro-B-type natriuretic peptide, and estimated glomerular filtration rate remained stable.
Conclusions
Favorable changes in 99mTc-PYP myocardial uptake were observed in participants on transthyretin stabilization therapy, whereas echocardiographic parameters and biomarkers remained stable. These results likely signify myocardial ATTR amyloid stabilization rather than amyloid burden regression. Further investigation is needed to understand the implications of these findings.
定量 99m锝-焦磷酸心肌摄取:转甲状腺素稳定疗法的变化
背景:定量锝-99m-焦磷酸心脏单光子发射计算机断层扫描(99mTc-PYP SPECT/CT)是估算经淀粉样蛋白心脏淀粉样变性(ATTR-CA)心肌负荷的一种新兴方法,但其在监测纵向变化方面的功效仍不确定。我们的目的是利用可视化和定量 99mTc-PYP SPECT/CT 研究经甲状腺素稳定疗法后心脏 ATTR 淀粉样蛋白负荷的纵向变化,并将其与心脏生物标志物和功能的变化联系起来:这项前瞻性纵向队列研究调查了23名接受转甲状腺素稳定治疗的ATTR-CA患者(中位2.6年)的99m锝-PYP SPECT/CT变化。定量分析包括左心室(LV)标准化摄取值(SUVmax、SUVmean)、心脏淀粉样蛋白活性(CAA;SUVmean*LV活性体积)和注射剂量百分比(%ID)(平均活性浓度*LV活性体积/注射活性),计算阈值为SPECT/CT上左心房血池活性浓度的>1.5倍。采用 Wilcoxon 符号秩检验分析配对连续变量和序数变量的纵向变化:结果:治疗后,视觉分级明显降低(P=0.003)。多项 99mTc-PYP 定量指标也显著下降:SUVmax(中位数为-0.75,p=0.011)、CAA(中位数为-406.6,p=0.011):在接受经甲状腺素稳定疗法的参与者中观察到了 99mTc-PYP 心肌摄取量的有利变化,而超声心动图参数和生物标志物则保持稳定。这些结果可能意味着心肌ATTR淀粉样蛋白稳定,而不是淀粉样蛋白负担减轻。要了解这些发现的意义,还需要进一步的研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.30
自引率
20.80%
发文量
249
审稿时长
4-8 weeks
期刊介绍:
Journal of Nuclear Cardiology is the only journal in the world devoted to this dynamic and growing subspecialty. Physicians and technologists value the Journal not only for its peer-reviewed articles, but also for its timely discussions about the current and future role of nuclear cardiology. Original articles address all aspects of nuclear cardiology, including interpretation, diagnosis, imaging equipment, and use of radiopharmaceuticals. As the official publication of the American Society of Nuclear Cardiology, the Journal also brings readers the latest information emerging from the Society''s task forces and publishes guidelines and position papers as they are adopted.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


