Changes in Red Cell Morphology and Haematological Laboratory Parameters Associated With Alectinib
Abstract
Background
Alectinib is a second-generation anaplastic lymphoma kinase (ALK) inhibitor indicated for ALK-mutated non-small-cell lung cancer. Recently, the association between alectinib and red cell morphological abnormalities has been reported in a few case series. This retrospective observational study aims to determine the frequency of occurrence of acanthocytosis in patients taking alectinib and to evaluate the red cell indices, biochemical markers of haemolysis and eosin-5-maleimide (EMA) binding assay results in patients receiving alectinib.
Methods
Patients who were on alectinib and had a complete blood count test performed in Queen Elizabeth Hospital Haematology Laboratory between 1 May 2021 and 31 August 2021 were included in the study. Haematological investigations that had been performed before and after the commencement of alectinib were reviewed.
Results
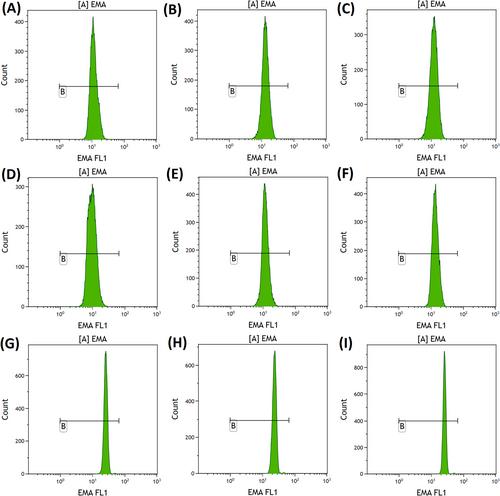
Fifty patients receiving alectinib were evaluated in this analysis. One hundred per cent of patients showed 3+ acanthocytes on the peripheral blood smears. Compared with the test results before starting alectinib, the post-alectinib blood tests showed a significantly lower haemoglobin concentration, red blood cell count and haematocrit; and a significantly higher mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration and red cell distribution width. All the tested patients showed a marked reduction in EMA mean channel fluorescence compared with normal control.
Conclusion
Our cohort revealed that alectinib caused significant acanthocytosis in all patients. Alectinib was also associated with changes in red cell indices and biochemical markers of haemolysis, compatible with a spherocytic and anisopoikilocytic morphology with haemolysis. Patients on alectinib had reduced EMA binding.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


