Gluten-Dependent Activation of CD4+ T Cells by MHC Class II–Expressing Epithelium
IF 25.7
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background & Aims
Intestinal epithelial cell (IEC) damage is a hallmark of celiac disease (CeD); however, its role in gluten-dependent T-cell activation is unknown. We investigated IEC-gluten-T-cell interactions in organoid monolayers expressing human major histocompatibility complex class II (HLA-DQ2.5), which facilitates gluten antigen recognition by CD4+ T cells in CeD.
Methods
Epithelial major histocompatibility complex class II (MHCII) was determined in active and treated CeD, and in nonimmunized and gluten-immunized DR3-DQ2.5 transgenic mice, lacking mouse MHCII molecules. Organoid monolayers from DR3-DQ2.5 mice were treated with or without interferon (IFN)-γ, and MHCII expression was evaluated by flow cytometry. Organoid monolayers and CD4+ T-cell co-cultures were incubated with gluten, predigested, or not by elastase-producing Pseudomonas aeruginosa or its lasB mutant. T-cell function was assessed based on proliferation, expression of activation markers, and cytokine release in the co-culture supernatants.
Results
Patients with active CeD and gluten-immunized DR3-DQ2.5 mice demonstrated epithelial MHCII expression. Organoid monolayers derived from gluten-immunized DR3-DQ2.5 mice expressed MHCII, which was upregulated by IFN-γ. In organoid monolayer T-cell co-cultures, gluten increased the proliferation of CD4+ T cells, expression of T-cell activation markers, and the release of interleukin-2, IFN-γ, and interleukin-15 in co-culture supernatants. Gluten metabolized by P aeruginosa, but not the lasB mutant, enhanced CD4+ T-cell proliferation and activation.
Conclusions
Gluten antigens are efficiently presented by MHCII-expressing IECs, resulting in the activation of gluten-specific CD4+ T cells, which is enhanced by gluten predigestion with microbial elastase. Therapeutics directed at IECs may offer a novel approach for modulating both adaptive and innate immunity in patients with CeD.
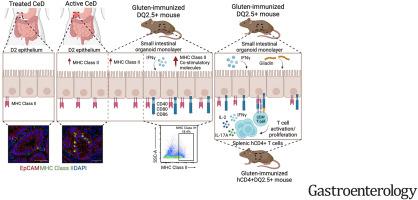
表达 MHC II 类上皮细胞对 CD4+ T 细胞的麸质依赖性激活。
背景和目的:肠上皮细胞(IEC)损伤是乳糜泻(CeD)的特征之一;然而,它在麸质依赖性T细胞活化中的作用尚不清楚。我们在表达人类 MHC II 类(HLA-DQ2.5)的类器官单层中研究了 IEC-谷蛋白-T 细胞之间的相互作用,这种作用有助于 CD4+ T 细胞识别麸质抗原:上皮 MHC II(MHCII)在活动性和经治疗的 CeD 以及未免疫和麸质免疫的 DR3-DQ2.5 转基因小鼠(缺乏小鼠 MHCII 分子)中进行了测定。用或不用 IFN-γ 处理 DR3-DQ2.5 小鼠的类器官单体,并用流式细胞术评估 MHCII 的表达。将类器官单层细胞和 CD4+ T 细胞共培养物与麸质培养物一起孵育,预消化或不消化产生弹性蛋白酶的铜绿假单胞菌或其 lasB 突变体。根据共培养上清中的增殖、活化标记物的表达和细胞因子的释放来评估T细胞的功能:结果:活动性CeD患者和麸质免疫DR3-DQ2.5小鼠均有上皮MHCII表达。来自麸质免疫 DR3-DQ2.5 小鼠的类器官单层细胞表达 MHCII,IFN-γ 对其有上调作用。在类器官单层-T 细胞共培养中,麸质增加了 CD4+ T 细胞的增殖、T 细胞活化标记物的表达以及共培养上清中 IL-2、IFN-γ 和 IL-15 的释放。铜绿假单胞菌代谢的麸质(而非 lasB 突变体)可增强 CD4+ T 细胞的增殖和活化:结论:麸质抗原可被表达 MHCII 的 IECs 有效呈现,导致麸质特异性 CD4+ T 细胞的活化,而用微生物弹性蛋白酶预消化麸质可增强这种活化。针对 IECs 的治疗方法可能为调节 CeD 患者的适应性免疫和先天性免疫提供了一种新方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Gastroenterology
医学-胃肠肝病学
CiteScore
45.60
自引率
2.40%
发文量
4366
审稿时长
26 days
期刊介绍:
Gastroenterology is the most prominent journal in the field of gastrointestinal disease. It is the flagship journal of the American Gastroenterological Association and delivers authoritative coverage of clinical, translational, and basic studies of all aspects of the digestive system, including the liver and pancreas, as well as nutrition.
Some regular features of Gastroenterology include original research studies by leading authorities, comprehensive reviews and perspectives on important topics in adult and pediatric gastroenterology and hepatology. The journal also includes features such as editorials, correspondence, and commentaries, as well as special sections like "Mentoring, Education and Training Corner," "Diversity, Equity and Inclusion in GI," "Gastro Digest," "Gastro Curbside Consult," and "Gastro Grand Rounds."
Gastroenterology also provides digital media materials such as videos and "GI Rapid Reel" animations. It is abstracted and indexed in various databases including Scopus, Biological Abstracts, Current Contents, Embase, Nutrition Abstracts, Chemical Abstracts, Current Awareness in Biological Sciences, PubMed/Medline, and the Science Citation Index.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


