Ring-closing metathesis of dialkenylcycloalkanes for the synthesis of fused bicycloalkanes and tricycloalkanes†
IF 4.6
Q2 MATERIALS SCIENCE, BIOMATERIALS
引用次数: 0
Abstract
Ring-closing metathesis of dialkenyldisilacycloalkane using the Grubbs catalyst, followed by hydrogenation, afforded a mixture of two disilabicycloalkanes and tetrasilatricycloalkanes. This method can synthesize a diastereomer mixture of disilabicycloalkanes with one differing alkyl side chain. The observed symmetries of the NMR spectra of anti-bicycloalkanes depend significantly on the length of the side chain. The findings may contribute to the molecular design of functional bicycloalkanes based on structural transformations of the stable forms.
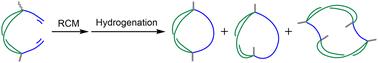
二烯基环烷烃的闭环偏析合成融合双环烷烃和三环烷烃。
使用 Grubbs 催化剂对二苯甲基二硅环烷烃进行闭环偏析,然后进行氢化反应,得到了两种二硅环烷烃和四硅环烷烃的混合物。这种方法可以合成具有一个不同烷基侧链的二苯并二环烷非对映异构体混合物。观察到的反双环烷烃核磁共振光谱的对称性在很大程度上取决于侧链的长度。这些发现可能有助于根据稳定形式的结构转变来设计功能性双环烷烃。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


