Electrochemical Reaction Kinetics at Constant Interfacial Potential
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Electrocatalysis has been recognized as one of the key technologies toward a carbon-neutral cycle of energy and substances. The rational design of electrocatalysts is undoubtedly the most important approach for accelerating the application of electrocatalysis. Computational screening of electrocatalysts based on thermodynamic evaluation is an efficient method for initially estimating their catalytic performances. However, the reaction rate at the electrochemical interface can be affected by many kinetic factors. Recently, we have developed a method for modeling potential/pH dependence in electrocatalysis, namely, electric field controlling constant potential (EFC-CP), which is much cheaper compared to the widely used grand canonical density functional theory calculations. This method can explicitly determine the evolution of real transition structures at varying potentials. As a result, both the chemical and electrostatic contributions to potential-dependent properties can be explicitly analyzed. Meanwhile, the change of the intermediate dipole along reaction coordinates can also be studied, which can reflect the pH dependence of the kinetic barrier. In this Perspective, we review the significant progress in understanding reaction kinetics in the application of electrochemical nitrogen fixation, ammonia synthesis, and denitrification. These insights can effectively help us understand the underlying physics of electrocatalytic reactions and improve the capability of catalyst design and modification. It is anticipated that the synergy between thermodynamic estimation and kinetic validation will enable the rational design of electrocatalysts in working condition.
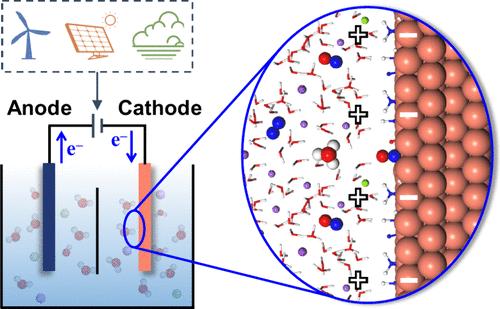
恒定界面电位下的电化学反应动力学
电催化技术已被公认为实现能源和物质碳中和循环的关键技术之一。合理设计电催化剂无疑是加速电催化应用的最重要方法。基于热力学评估对电催化剂进行计算筛选是初步估算其催化性能的有效方法。然而,电化学界面的反应速率会受到许多动力学因素的影响。最近,我们开发了一种电催化中电位/pH 依赖性的建模方法,即电场控制恒定电位(EFC-CP),与广泛使用的大规范密度泛函理论计算相比,该方法成本更低。这种方法可以明确确定不同电位下真实过渡结构的演变。因此,可以明确分析化学和静电对电位相关性质的贡献。同时,还可以研究中间偶极沿反应坐标的变化,这可以反映动力学势垒的 pH 依赖性。在本《视角》中,我们回顾了在电化学固氮、氨合成和反硝化应用中理解反应动力学的重大进展。这些见解能有效帮助我们理解电催化反应的基本物理原理,并提高催化剂设计和改性的能力。预计热力学估算和动力学验证之间的协同作用将有助于合理设计工作状态下的电催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


