Screening of protein-based inhibitors for the intracellular domain of epidermal growth factor receptor by directed evolution using the yeast Gγ recruitment system
IF 2.3
4区 生物学
Q3 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Protein-based therapeutics, including antibodies and antibody-like-proteins, have increasingly attracted attention due to their high specificity compared to small-molecular drugs. The Gγ recruitment system, one of the in vivo yeast two-hybrid systems for detecting protein–protein interactions, has been previously developed using yeast signal transduction machinery. In this study, we modified the Gγ recruitment system to screen the protein mutants that efficiently bind to the intracellular domain of the epidermal growth factor receptor L858R mutant (cytoEGFRL858R). Using the modified platform, we performed in vivo directed evolution for growth factor receptor-bound protein 2 (Grb2) and its truncated variant containing only the Src-homology 2 (SH2) domain, successfully identifying several mutants that more strongly bound to cytoEGFRL858R than their parental proteins. Some of them contained novel beneficial mutations (F108Y and Q144H) and specifically bound to the recombinant cytosolic phosphorylated EGFR in vitro, highlighting the utility of the evolutionary platform.
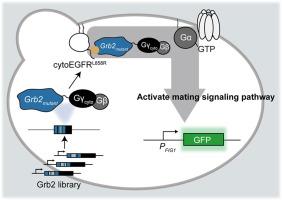
利用酵母 Gγ 招募系统,通过定向进化筛选表皮生长因子受体胞内结构域的蛋白抑制剂。
与小分子药物相比,以蛋白质为基础的疗法(包括抗体和抗体样蛋白)具有高度特异性,因此越来越受到关注。Gγ 招募系统是体内酵母双杂交系统之一,用于检测蛋白质与蛋白质之间的相互作用。在本研究中,我们对 Gγ 招募系统进行了改进,以筛选能与表皮生长因子受体 L858R 突变体(cytoEGFRL858R)胞内结构域有效结合的蛋白质突变体。利用改进后的平台,我们对生长因子受体结合蛋白2(Grb2)及其仅含有Src-homology 2(SH2)结构域的截短变体进行了体内定向进化,成功鉴定出几种突变体,它们与cytoEGFRL858R的结合比亲代蛋白更强。其中一些突变体含有新的有益突变(F108Y和Q144H),并在体外与重组的细胞磷酸化表皮生长因子受体特异性结合,凸显了进化平台的实用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of bioscience and bioengineering
生物-生物工程与应用微生物
CiteScore
5.90
自引率
3.60%
发文量
144
审稿时长
51 days
期刊介绍:
The Journal of Bioscience and Bioengineering is a research journal publishing original full-length research papers, reviews, and Letters to the Editor. The Journal is devoted to the advancement and dissemination of knowledge concerning fermentation technology, biochemical engineering, food technology and microbiology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


