Mechanisms of antibody-dependent enhancement of infectious disease
IF 67.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
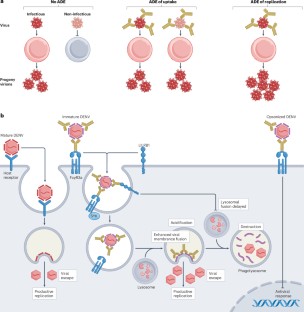
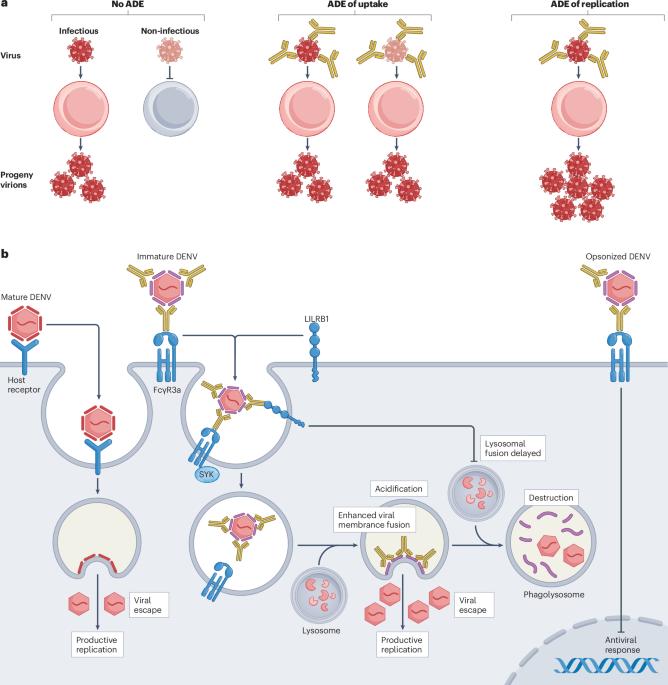
Antibody-dependent enhancement (ADE) of infectious disease is a phenomenon whereby host antibodies increase the severity of an infection. It is well established in viral infections but ADE also has an underappreciated role during bacterial, fungal and parasitic infections. ADE can occur during both primary infections and re-infections with the same or a related pathogen; therefore, understanding the underlying mechanisms of ADE is critical for understanding the pathogenesis and progression of many infectious diseases. Here, we review the four distinct mechanisms by which antibodies increase disease severity during an infection. We discuss the most established mechanistic explanation for ADE, where cross-reactive, disease-enhancing antibodies bound to pathogens interact with Fc receptors, thereby enhancing pathogen entry or replication, ultimately increasing the total pathogen load. Additionally, we explore how some pathogenic antibodies can shield bacteria from complement-dependent killing, thereby enhancing bacterial survival. We interrogate the molecular mechanisms by which antibodies can amplify inflammation to drive severe disease, even in the absence of increased pathogen replication. We also examine emerging roles for autoantibodies in enhancing the pathogenesis of infectious diseases. Finally, we discuss how we can leverage these insights to improve vaccine design and future treatments for infectious diseases. This Review discusses the different mechanisms of antibody-dependent enhancement (ADE) of infectious disease, including how antibodies can increase the pathogen load, protect bacteria from the immune system and amplify inflammation. The authors also highlight the role of autoantibodies and consider how a better understanding of ADE can be used to improve vaccines and treatments for infectious diseases.

抗体依赖性增强传染性疾病的机制
传染性疾病的抗体依赖性增强(ADE)是宿主抗体增加感染严重程度的一种现象。这种现象在病毒感染中已得到公认,但在细菌、真菌和寄生虫感染中,ADE 的作用也未得到充分重视。ADE 既可发生在原发感染中,也可发生在同一病原体或相关病原体的再感染中;因此,了解 ADE 的基本机制对于了解许多感染性疾病的发病机制和进展至关重要。在此,我们回顾了抗体在感染期间增加疾病严重性的四种不同机制。我们讨论了 ADE 最成熟的机制解释,即与病原体结合的交叉反应性疾病增强抗体与 Fc 受体相互作用,从而增强病原体的进入或复制,最终增加病原体的总负荷。此外,我们还探讨了一些致病性抗体是如何使细菌免于补体依赖性杀灭,从而提高细菌存活率的。我们探究了抗体在没有增加病原体复制的情况下扩大炎症以导致严重疾病的分子机制。我们还研究了自身抗体在增强传染病发病机制方面的新作用。最后,我们将讨论如何利用这些见解来改进疫苗设计和未来的传染病治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Immunology
医学-免疫学
CiteScore
93.40
自引率
0.40%
发文量
131
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Immunology is a journal that provides comprehensive coverage of all areas of immunology, including fundamental mechanisms and applied aspects. It has two international standard serial numbers (ISSN): 1474-1733 for print and 1474-1741 for online. In addition to review articles, the journal also features recent developments and new primary papers in the field, as well as reflections on influential people, papers, and events in the development of immunology. The subjects covered by Nature Reviews Immunology include allergy and asthma, autoimmunity, antigen processing and presentation, apoptosis and cell death, chemokines and chemokine receptors, cytokines and cytokine receptors, development and function of cells of the immune system, haematopoiesis, infection and immunity, immunotherapy, innate immunity, mucosal immunology and the microbiota, regulation of the immune response, signalling in the immune system, transplantation, tumour immunology and immunotherapy, and vaccine development.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


