Selection of antibody-binding covalent aptamers
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Aptamers are oligonucleotides with antibody-like binding function, selected from large combinatorial libraries. In this study, we modified a DNA aptamer library with N-hydroxysuccinimide esters, enabling covalent conjugation with cognate proteins. We selected for the ability to bind to mouse monoclonal antibodies, resulting in the isolation of two distinct covalent binding motifs. The covalent aptamers are specific for the Fc region of mouse monoclonal IgG1 and are cross-reactive with mouse IgG2a and other IgGs. Investigation into the covalent conjugation of the aptamers revealed a dependence on micromolar concentrations of Cu2+ ions which can be explained by residual catalyst remaining after modification of the aptamer library. The aptamers were successfully used as adapters in the formation of antibody-oligonucleotide conjugates (AOCs) for use in detection of HIV protein p24 and super-resolution imaging of actin. This work introduces a new method for the site-specific modification of native monoclonal antibodies and may be useful in applications requiring AOCs or other antibody conjugates. Site-specific conjugation of oligonucleotides and native proteins remains challenging. Here, the authors select covalent DNA aptamers from a library modified with N-hydroxysuccinimide esters, and show their application in the formation of antibody–oligonucleotide conjugates for protein detection.
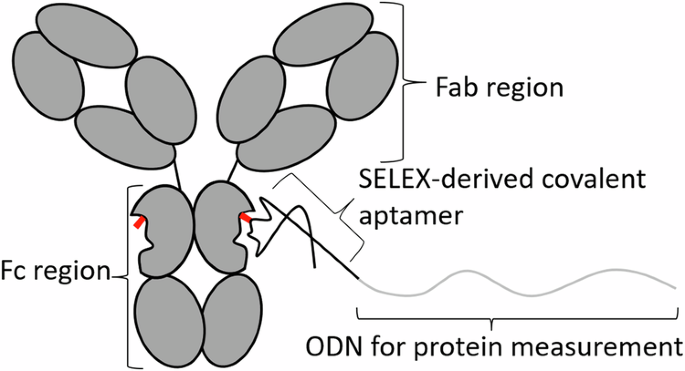
筛选抗体结合型共价配合物。
适配体是从大型组合文库中筛选出来的具有类似抗体结合功能的寡核苷酸。在这项研究中,我们用N-羟基琥珀酰亚胺酯修饰了DNA适配体文库,使其能与同源蛋白共价结合。我们选择了与小鼠单克隆抗体结合的能力,从而分离出两种不同的共价结合基团。共价配合物对小鼠单克隆 IgG1 的 Fc 区具有特异性,与小鼠 IgG2a 和其他 IgG 具有交叉反应。对适配体共价结合的研究发现,这种共价结合与微摩尔浓度的 Cu2+ 离子有关,其原因可能是适配体文库修饰后残留的催化剂。这些适配体被成功地用作抗体-寡核苷酸共轭物(AOCs)的适配体,用于检测 HIV 蛋白 p24 和肌动蛋白的超分辨率成像。这项工作介绍了一种对原生单克隆抗体进行特定位点修饰的新方法,可能会在需要 AOC 或其他抗体共轭物的应用中发挥作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


