Identification of Novel Potential Predisposing Variants in Familial Acute Myeloid Leukemia
Abstract
Background
Myeloid neoplasms, including acute myeloid leukemia, have been traditionally among the less investigated cancer types concerning germline predisposition. Indeed, myeloid neoplasms with germline predisposition are challenging to identify because often display similar clinical and morphological characteristics of sporadic cases and have similar age at diagnosis. However, a misidentifications of familiarity in myeloid neoplasms have a critical impact on clinical management both for the carriers and their relatives.
Aims
We conducted a family segregation study, in order to identify novel cancer predisposing genes in myeloid neoplasms and classify novel identified variants.
Methods and Results
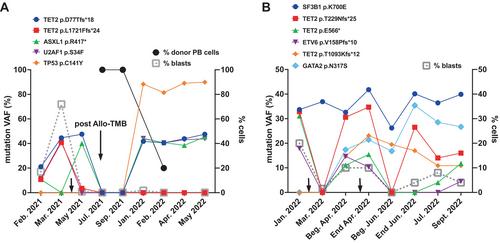
We performed a thorough genomic analysis using a large custom gene panel (256 genes), the Myelo-Panel, targeted on cancer predisposing genes. In particular, we assessed both germline and somatic variants in four families, each with two siblings, who developed hematological neoplasms: seven acute myeloid leukemia and one Philadelphia-positive chronic myeloid leukemia. In each family, we identified at least one novel potentially predisposing variant, affecting also genes not included in the current European LeukemiaNet guidelines for AML management. Moreover, we suggest reclassification of two germline variants as pathogenic: likely pathogenic p.S21Tfs*139 in CEPBA and VUS p.K392Afs*66 in DDX41.
Conclusion
We believe that predisposition to hematological neoplasms is still underestimated and particularly difficult to diagnosed. Considering that misidentification of familiarity in myeloid neoplasms have a critical impact on the clinical management both for the carriers and their relatives, our study highlights the importance of revision, in this clinical context, of clinical practices that should include thorough reconstruction of family history and in-depth genetic testing.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


