Iron-catalyzed synthesis of substituted 3-arylquinolin-2(1H)-ones via an intramolecular dehydrogenative coupling of amido-alcohols†
IF 4.6
Q2 MATERIALS SCIENCE, BIOMATERIALS
引用次数: 0
Abstract
Here we report an iron-complex-catalyzed synthesis of various mono- and di-substituted quinolin-2(1H)-ones achieved via the intramolecular acceptorless dehydrogenative cyclization of amido-alcohols. This approach for the synthesis of N-heterocycles has provided access to underdescribed disubstituted quinolinones and represents an alternative to the well-known palladium-catalyzed coupling reactions.
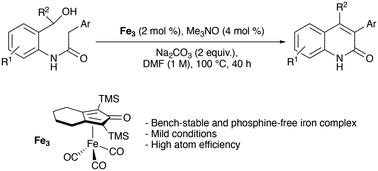
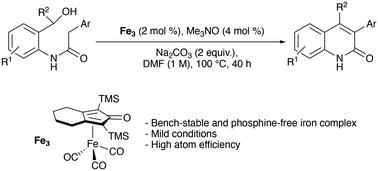
通过氨基醇分子内脱氢偶联铁催化合成取代的 3-芳基喹啉-2(1H)-酮。
在此,我们报告了一种铁络合物催化的合成方法,该方法通过氨基醇的分子内无受体脱氢环化实现了各种单取代和双取代喹啉-2(1H)-酮的合成。这种合成 N-杂环的方法提供了获得未充分描述的二取代喹啉酮的途径,是众所周知的钯催化偶联反应的替代方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


