Algicidal bacteria-derived membrane vesicles as shuttles mediating cross-kingdom interactions between bacteria and algae
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Bacterial membrane vesicles (BMVs) are crucial biological vehicles for facilitating interspecies and interkingdom interactions. However, the extent and mechanisms of BMV involvement in bacterial-algal communication remain elusive. This study provides evidence of BMVs delivering cargos to targeted microalgae. Membrane vesicles (MVs) from Chitinimonas prasina LY03 demonstrated an algicidal profile similar to strain LY03. Further investigation revealed Tambjamine LY2, an effective algicidal compound, selectively packaged into LY03-MVs. Microscopic imaging demonstrated efficient delivery of Tambjamine LY2 to microalgae Heterosigma akashiwo and Thalassiosira pseudonana through membrane fusion. In addition, the study demonstrated the versatile cargo delivery capabilities of BMVs to algae, including the transfer of MV-carried nucleic acids into algal cells and the revival of growth in iron-depleted microalgae by MVs. Collectively, our findings reveal a previously unknown mechanism by which algicidal bacteria store hydrophobic algicidal compounds in MVs to trigger target microalgae death and highlight BMV potency in understanding and engineering bacterial-algae cross-talk.
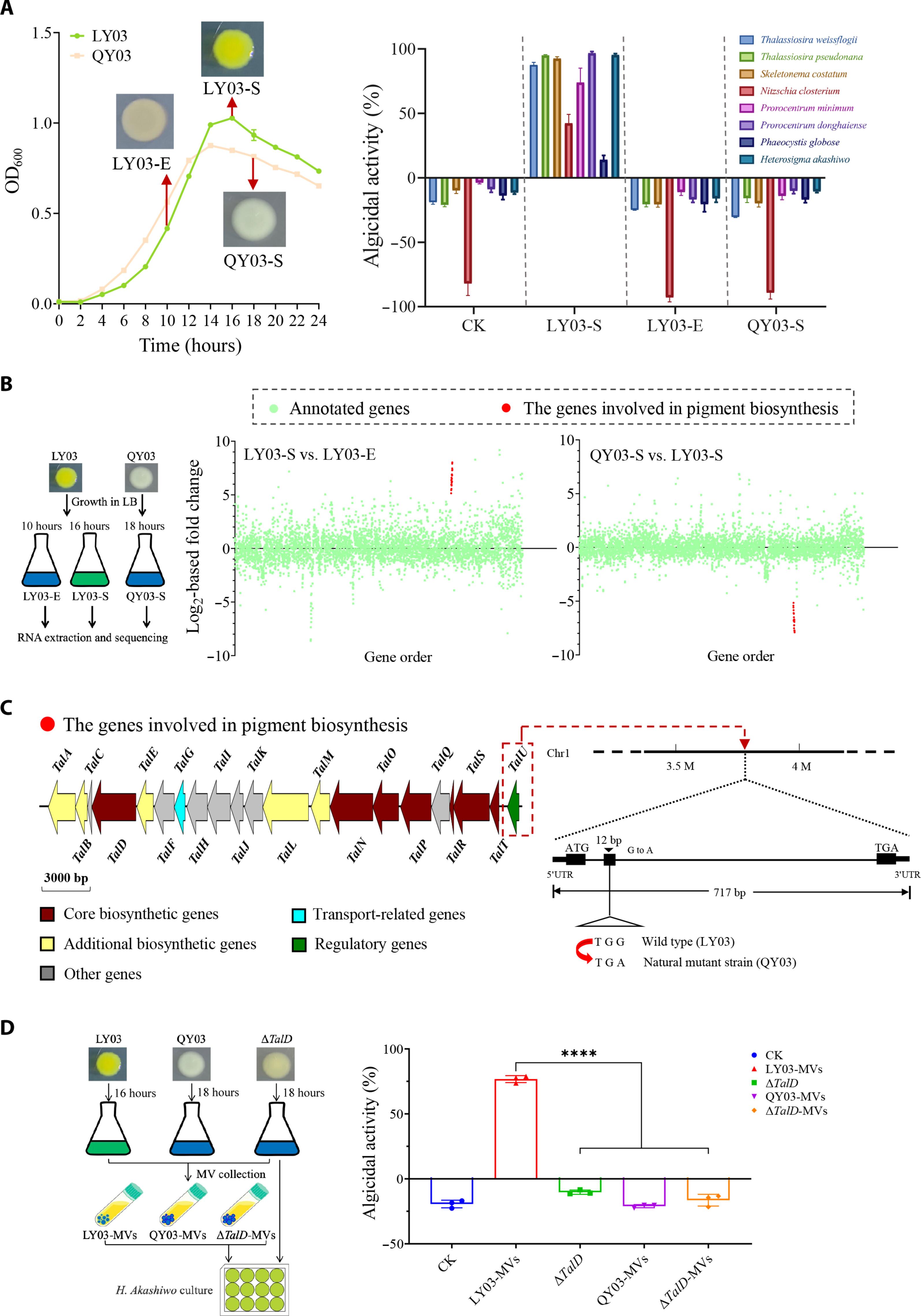
杀藻细菌衍生的膜囊泡是介导细菌和藻类之间跨领域互动的穿梭器。
细菌膜囊(BMV)是促进物种间和生物界间相互作用的重要生物载体。然而,细菌膜囊泡参与细菌与藻类交流的程度和机制仍然难以捉摸。本研究提供了 BMV 向目标微藻运送货物的证据。Chitinimonas prasina LY03 的膜囊(MVs)显示出与菌株 LY03 相似的杀藻特性。进一步研究发现,一种有效的杀藻化合物 Tambjamine LY2 被选择性地包装到 LY03-MVs 中。显微成像显示,Tambjamine LY2 通过膜融合有效地输送到微藻 Heterosigma akashiwo 和 Thalassiosira pseudonana。此外,该研究还证明了 BMV 对藻类的多功能货物输送能力,包括将 MV 所携带的核酸转移到藻类细胞中,以及通过 MV 恢复缺铁微藻的生长。总之,我们的研究结果揭示了一种以前未知的机制,即杀藻细菌将疏水性杀藻化合物储存在中空分子中,从而引发目标微藻死亡,并突出了中空分子在理解和设计细菌-藻类交叉对话方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


