Hydrogenation of CO2 to CH3OH on the Cu–ZnO–SrTiO3 Catalysts: The Electronic Metal–Support Interaction Induces Oxygen Vacancy Generation
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
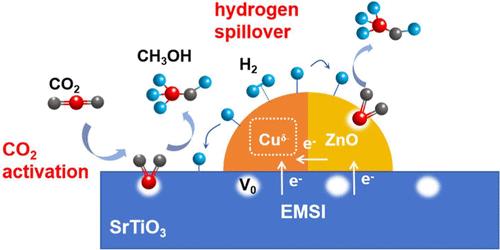
With the massive burning of fossil energy sources, the greenhouse effect is increasingly significant, and the reduction of the CO2 concentration in the atmosphere is imminent. In this work, Cu–ZnO–SrTiO3 catalysts with different Cu–Zn loadings and Cu/Zn atomic ratios were prepared by the deposition–coprecipitation method using n-type semiconductor SrTiO3 with a perovskite structure as a support for the CO2 hydrogenation to methanol process. In situ XPS, in situ CO–DRIFTS, electron paramagnetic resonance (EPR), and UV confirmed that electron transfer from the supports to Cu is the intrinsic nature of the electronic metal–support interaction between Cu and the supports, resulting in oxygen vacancy generation. Electron transfer is attributed to the difference in the Fermi energy levels of the metal and the supports, which in turn form Schottky–Mott junctions. EPR, CO2-TPD, and catalytic activity illustrated that oxygen vacancies (Ov) in the supports (SrTiO3 and ZnO) enhance the activation of CO2. H2-TPD demonstrated that Cuδ− species in contact with the supports facilitate hydrogen spillover. Cuδ−–Ov at the interface may be the active sites of catalysts. In addition, in situ XRD verified that the larger the electron transfer, the smaller the corresponding Cu particle diameter.
在 Cu-ZnO-SrTiO3 催化剂上将 CO2 加氢转化为 CH3OH:电子金属-支撑相互作用诱导氧空位生成
随着化石能源的大量燃烧,温室效应日益显著,降低大气中的二氧化碳浓度迫在眉睫。本研究以具有包晶结构的 n 型半导体 SrTiO3 为载体,采用沉积-沉淀法制备了不同 Cu-Zn 负载和 Cu/Zn 原子比的 Cu-ZnO-SrTiO3 催化剂,用于 CO2 加氢制甲醇过程。原位 XPS、原位 CO-DRIFTS、电子顺磁共振(EPR)和紫外线证实,电子从支撑体转移到铜是铜与支撑体之间电子金属-支撑体相互作用的内在性质,从而导致氧空位的产生。电子转移是由于金属和支撑物的费米能级不同,进而形成了肖特基-莫特结。EPR、CO2-TPD 和催化活性表明,支撑物(SrTiO3 和 ZnO)中的氧空位(Ov)增强了 CO2 的活化。H2-TPD 表明,与支撑物接触的 Cuδ- 物种促进了氢的溢出。界面上的 Cuδ--Ov 可能是催化剂的活性位点。此外,原位 XRD 验证了电子转移越大,相应的铜颗粒直径越小。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


