Age-related epithelial defects limit thymic function and regeneration
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
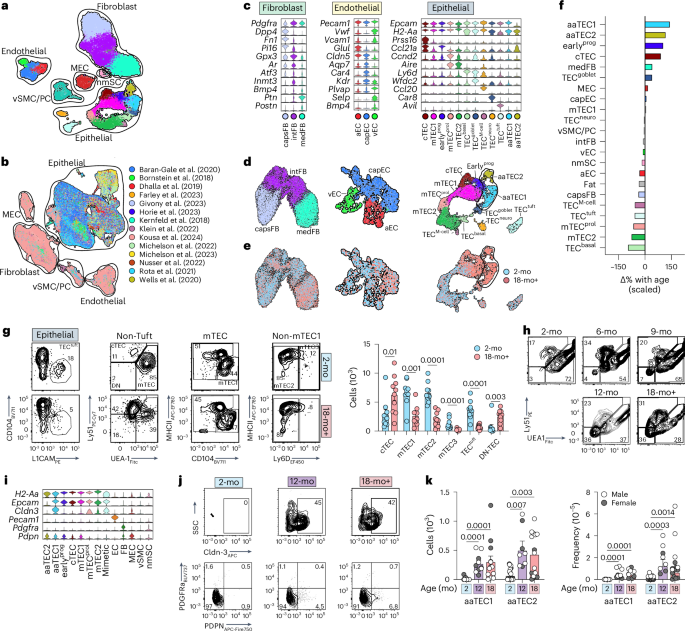
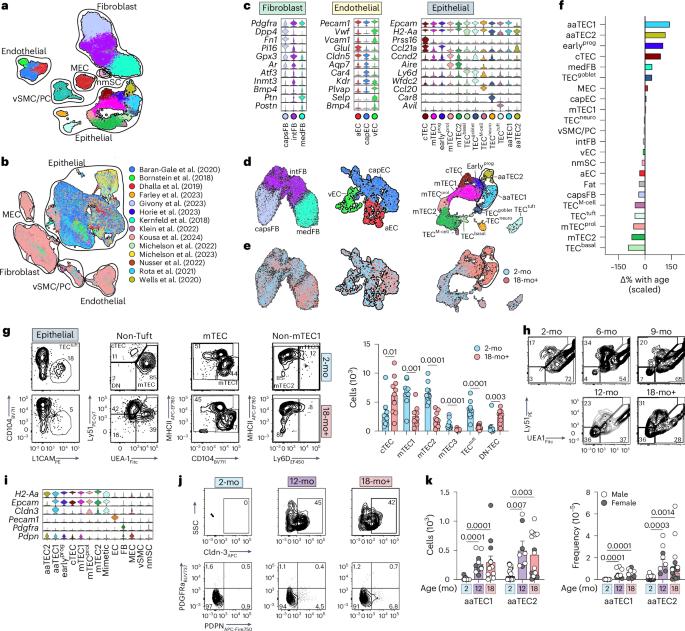
The thymus is essential for establishing adaptive immunity yet undergoes age-related involution that leads to compromised immune responsiveness. The thymus is also extremely sensitive to acute insult and although capable of regeneration, this capacity declines with age for unknown reasons. We applied single-cell and spatial transcriptomics, lineage-tracing and advanced imaging to define age-related changes in nonhematopoietic stromal cells and discovered the emergence of two atypical thymic epithelial cell (TEC) states. These age-associated TECs (aaTECs) formed high-density peri-medullary epithelial clusters that were devoid of thymocytes; an accretion of nonproductive thymic tissue that worsened with age, exhibited features of epithelial-to-mesenchymal transition and was associated with downregulation of FOXN1. Interaction analysis revealed that the emergence of aaTECs drew tonic signals from other functional TEC populations at baseline acting as a sink for TEC growth factors. Following acute injury, aaTECs expanded substantially, further perturbing trophic regeneration pathways and correlating with defective repair of the involuted thymus. These findings therefore define a unique feature of thymic involution linked to immune aging and could have implications for developing immune-boosting therapies in older individuals. Here the authors identify age-associated changes in the epithelial cell compartment of the thymus that form high-density nonproductive microenvironmental niches that contribute toward thymic involution and inhibit its repair following injury.
与年龄有关的上皮缺陷限制了胸腺功能和再生
胸腺对建立适应性免疫至关重要,但随着年龄的增长,胸腺会逐渐萎缩,导致免疫反应能力下降。胸腺对急性损伤也极为敏感,虽然有再生能力,但这种能力会随着年龄的增长而下降,原因不明。我们应用单细胞和空间转录组学、品系追踪和高级成像技术来确定非造血基质细胞与年龄相关的变化,并发现了两种非典型胸腺上皮细胞(TEC)状态的出现。这些与年龄相关的胸腺上皮细胞(aaTECs)形成了高密度的髓周上皮细胞簇,其中没有胸腺细胞;这是一种非生产性胸腺组织的增生,随着年龄的增长而恶化,表现出上皮细胞向间质转化的特征,并与 FOXN1 的下调有关。相互作用分析表明,aaTEC 的出现从基线的其他功能性 TEC 群体中吸取了营养信号,成为 TEC 生长因子的汇集点。急性损伤后,aaTECs 大量扩增,进一步扰乱了营养再生途径,并与内陷胸腺的缺陷修复有关。因此,这些发现确定了与免疫衰老有关的胸腺内陷的一个独特特征,并可能对开发老年人免疫增强疗法产生影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


