Gaucher disease provides a unique window into Parkinson disease pathogenesis
IF 28.2
1区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
Abstract
An exciting development in the field of neurodegeneration is the association between the rare monogenic disorder Gaucher disease and the common complex disorder Parkinson disease (PD). Gaucher disease is a lysosomal storage disorder resulting from an inherited deficiency of the enzyme glucocerebrosidase, encoded by GBA1, which hydrolyses the glycosphingolipids glucosylceramide and glucosylsphingosine. The observation of parkinsonism in a rare subgroup of individuals with Gaucher disease first directed attention to the role of glucocerebrosidase deficiency in the pathogenesis of PD. PD occurs more frequently in people heterozygous for Gaucher GBA1 mutations, and 3–25% of people with Parkinson disease carry a GBA1 variant. However, only a small percentage of individuals with GBA1 variants develop parkinsonism, suggesting that the penetrance is low. Despite over a decade of intense research in this field, including clinical and radiological evaluations, genetic studies and investigations using model systems, the mechanism underlying GBA1-PD is still being pursued. Insights from this association have emphasized the role of lysosomal pathways in parkinsonism. Furthermore, different therapeutic strategies considered or developed for Gaucher disease can now inform drug development for PD. The association between the rare, monogenic lysosomal storage disorder Gaucher disease and Parkinson disease has provided insights into the pathogenesis of this far more common neurodegenerative disease. Here, Sidransky and colleagues review the knowledge gained from decades of Gaucher disease research and explore the relationship between GBA1 and parkinsonism.
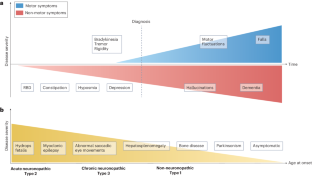
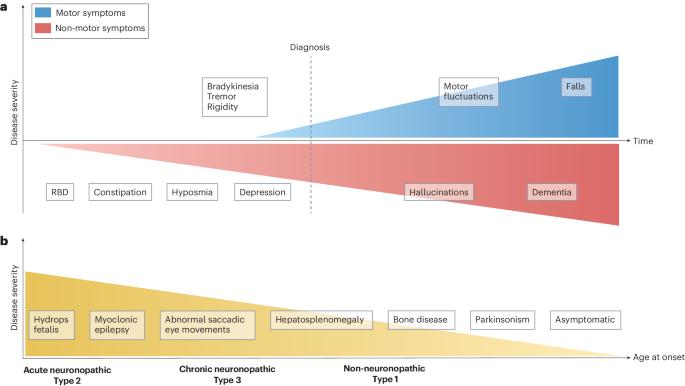
戈谢病为了解帕金森病的发病机制提供了一个独特的窗口。
神经变性领域一个令人兴奋的进展是罕见的单基因疾病戈谢病与常见的复杂性疾病帕金森病(PD)之间的联系。戈谢病是一种溶酶体储积症,是由于遗传性葡萄糖脑苷脂酶(由 GBA1 编码)缺乏所致,该酶可水解糖磷脂葡萄糖酰胺和葡萄糖鞘氨醇。在一个罕见的戈谢病亚群体中观察到帕金森病,首次将人们的注意力引向葡萄糖脑苷脂酶缺乏症在帕金森病发病机制中的作用。帕金森病多发于戈谢病 GBA1 基因突变的杂合子患者,3-25% 的帕金森病患者携带 GBA1 基因变异。然而,只有一小部分 GBA1 变体携带者会患上帕金森病,这表明帕金森病的发病率很低。尽管在这一领域进行了十多年的深入研究,包括临床和放射学评估、遗传学研究和使用模型系统的调查,但 GBA1-PD 的内在机制仍在研究之中。从这一关联中获得的启示强调了溶酶体通路在帕金森病中的作用。此外,针对戈谢病考虑或开发的不同治疗策略现在可以为帕金森病的药物开发提供参考。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Neurology
医学-临床神经学
CiteScore
29.90
自引率
0.80%
发文量
138
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Neurology aims to be the premier source of reviews and commentaries for the scientific and clinical communities we serve. We want to provide an unparalleled service to authors, referees, and readers, and we work hard to maximize the usefulness and impact of each article. The journal publishes Research Highlights, Comments, News & Views, Reviews, Consensus Statements, and Perspectives relevant to researchers and clinicians working in the field of neurology. Our broad scope ensures that the work we publish reaches the widest possible audience. Our articles are authoritative, accessible, and enhanced with clearly understandable figures, tables, and other display items. This page gives more detail about the aims and scope of the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


