Does HPV vaccination during periconceptional or gestational period increase the risk of adverse pregnancy outcomes?—An updated systematic review and meta-analysis based on timing of vaccination
Abstract
Introduction
The human papillomavirus (HPV) vaccine is crucial in preventing cervical cancer, and a significant number of women in 135 countries worldwide may have unknowingly received the vaccine during peri-pregnancy or pregnancy due to a lack of regular pregnancy testing. Previous studies on the safety of pregnancy outcomes with vaccination before and after pregnancy have not reached definitive conclusions. Thus, we subdivided the vaccination time frame and conducted an updated study to further examine whether exposure to the HPV vaccine during pregnancy or the periconceptional period increases the likelihood of adverse pregnancy outcomes.
Material and Methods
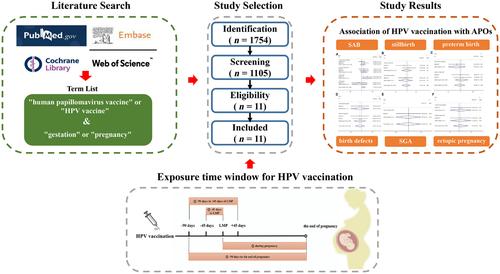
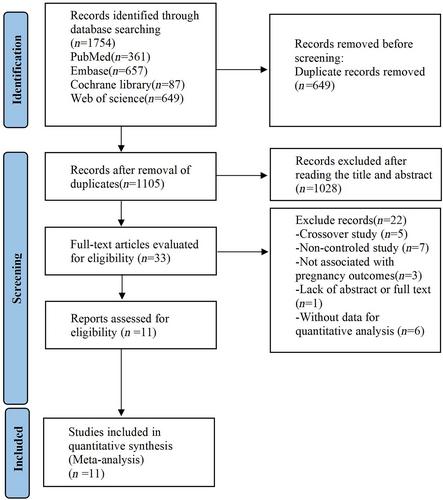
The clinical trials and cohort studies published before August 1, 2023, were retrieved from PubMed, Embase, Web of Science, and Cochrane Central Register of Controlled Trials. The Newcastle–Ottawa Scale and Cochrane risk of bias assessment tool were adopted to evaluate the risk of bias in the included studies. In addition, the quality assessment was carried out using the Review Manager 5.4 Software, and a meta-analysis was conducted using the Stata 16 Software.
Results
Eleven studies were located. The results showed that receiving 4vHPV during the periconceptional or gestational period had no relationship with an increased risk of spontaneous abortion, stillbirth, preterm birth, birth defects, small for gestational age, and ectopic pregnancy. Neither receiving 2vHPV nor 9vHPV was associated with a higher risk of stillbirth, preterm birth, birth defects, small for gestational age, and ectopic pregnancy; however, receiving 2vHPV during the period from 45 days before last menstrual period (LMP) to LMP and 9vHPV during the period from 90 days before LMP to 45 days after LMP seemed to be related to an increased risk of spontaneous abortion (RR = 1.59, 95% CI: 1.04–2.45, RR = 2.04, 95% CI: 1.28–3.24).
Conclusions
In conclusion, the likelihood of an elevated risk of spontaneous abortion caused by HPV vaccination during the periconceptional or gestational period could not be completely ruled out. Given the lack of evidence, further research is needed to examine the effect of HPV vaccination on spontaneous abortion.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


