Mechanisms of autophagy–lysosome dysfunction in neurodegenerative diseases
IF 81.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Autophagy is a lysosome-based degradative process used to recycle obsolete cellular constituents and eliminate damaged organelles and aggregate-prone proteins. Their postmitotic nature and extremely polarized morphologies make neurons particularly vulnerable to disruptions caused by autophagy–lysosomal defects, especially as the brain ages. Consequently, mutations in genes regulating autophagy and lysosomal functions cause a wide range of neurodegenerative diseases. Here, we review the role of autophagy and lysosomes in neurodegenerative diseases such as Alzheimer disease, Parkinson disease and frontotemporal dementia. We also consider the strong impact of cellular ageing on lysosomes and autophagy as a tipping point for the late-age emergence of related neurodegenerative disorders. Many of these diseases have primary defects in autophagy, for example affecting autophagosome formation, and in lysosomal functions, especially pH regulation and calcium homeostasis. We have aimed to provide an integrative framework for understanding the central importance of autophagic–lysosomal function in neuronal health and disease. The autophagy–lysosome pathway eliminates damaged organelles and aggregation-prone proteins, which is particularly important in neurons, where clearance of such substrates is restricted. Autophagy or lysosome deficiencies, often exacerbated by ageing, impact neuronal function and cause neurodegenerative diseases such as Alzheimer disease or Parkinson disease.
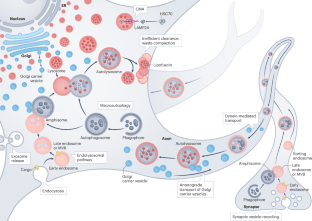
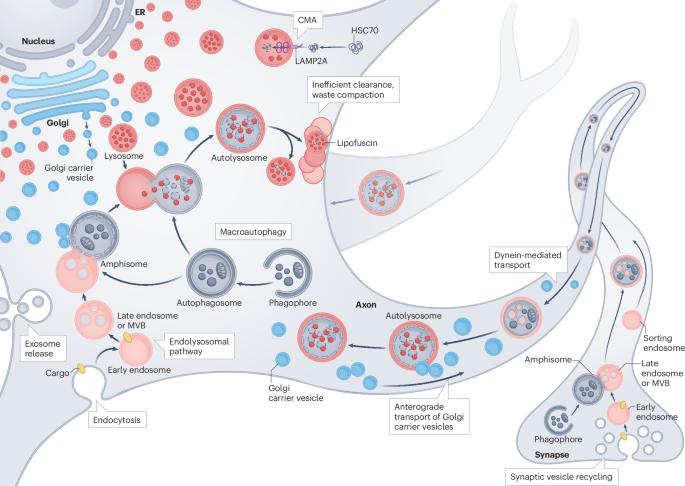
神经退行性疾病中自噬-溶酶体功能障碍的机制
自噬是一种基于溶酶体的降解过程,用于回收过时的细胞成分,消除受损的细胞器和易聚集的蛋白质。神经元的有丝分裂后特性和极度极化的形态使其特别容易受到自噬-溶酶体缺陷造成的破坏,尤其是在大脑衰老的过程中。因此,调控自噬和溶酶体功能的基因突变会导致多种神经退行性疾病。在此,我们回顾了自噬和溶酶体在阿尔茨海默病、帕金森病和额颞叶痴呆症等神经退行性疾病中的作用。我们还将细胞老化对溶酶体和自噬的强烈影响视为相关神经退行性疾病晚期出现的临界点。这些疾病中有许多都存在自噬的主要缺陷,例如影响自噬体的形成,以及溶酶体功能,尤其是 pH 值调节和钙平衡。我们旨在提供一个综合框架,以了解自噬-溶酶体功能在神经元健康和疾病中的核心重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
173.60
自引率
0.50%
发文量
118
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Molecular Cell Biology is a prestigious journal that aims to be the primary source of reviews and commentaries for the scientific communities it serves. The journal strives to publish articles that are authoritative, accessible, and enriched with easily understandable figures, tables, and other display items. The goal is to provide an unparalleled service to authors, referees, and readers, and the journal works diligently to maximize the usefulness and impact of each article. Nature Reviews Molecular Cell Biology publishes a variety of article types, including Reviews, Perspectives, Comments, and Research Highlights, all of which are relevant to molecular and cell biologists. The journal's broad scope ensures that the articles it publishes reach the widest possible audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


