Structural basis for linker histone H5–nucleosome binding and chromatin fiber compaction
IF 25.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
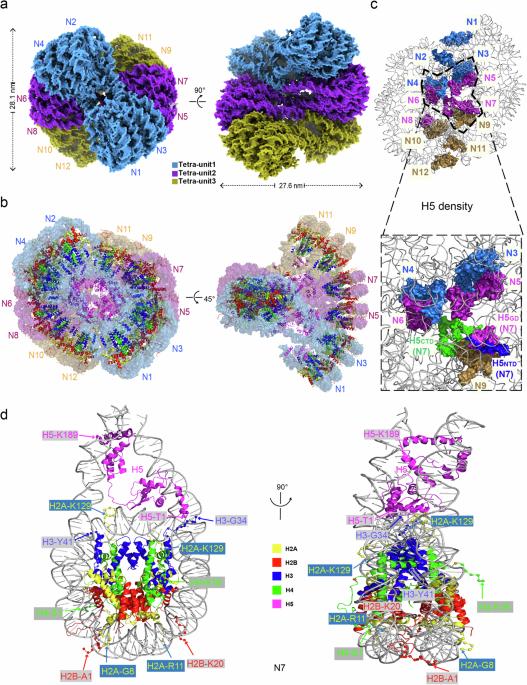
The hierarchical packaging of chromatin fibers plays a critical role in gene regulation. The 30-nm chromatin fibers, a central-level structure bridging nucleosomal arrays to higher-order organizations, function as the first level of transcriptional dormant chromatin. The dynamics of 30-nm chromatin fiber play a crucial role in biological processes related to DNA. Here, we report a 3.6-angstrom resolution cryogenic electron microscopy structure of H5-bound dodecanucleosome, i.e., the chromatin fiber reconstituted in the presence of linker histone H5, which shows a two-start left-handed double helical structure twisted by tetranucleosomal units. An atomic structural model of the H5-bound chromatin fiber, including an intact chromatosome, is built, which provides structural details of the full-length linker histone H5, including its N-terminal domain and an HMG-motif-like C-terminal domain. The chromatosome structure shows that H5 binds the nucleosome off-dyad through a three-contact mode in the chromatin fiber. More importantly, the H5-chromatin structure provides a fine molecular basis for the intra-tetranucleosomal and inter-tetranucleosomal interactions. In addition, we systematically validated the physiological functions and structural characteristics of the tetranucleosomal unit through a series of genetic and genomic studies in Saccharomyces cerevisiae and in vitro biophysical experiments. Furthermore, our structure reveals that multiple structural asymmetries of histone tails confer a polarity to the chromatin fiber. These findings provide structural and mechanistic insights into how a nucleosomal array folds into a higher-order chromatin fiber with a polarity in vitro and in vivo.

连接组蛋白 H5-核小体结合和染色质纤维压实的结构基础
染色质纤维的分级包装在基因调控中起着至关重要的作用。30纳米染色质纤维是连接核糖体阵列和高阶组织的中心层结构,是转录休眠染色质的第一层。30-nm 染色质纤维的动态在与 DNA 相关的生物过程中起着至关重要的作用。在此,我们报告了一个 3.6 埃的 H5 结合十二核小体低温电子显微镜结构,即在连接子组蛋白 H5 存在的情况下重组的染色质纤维,它显示了一个由四核小体单位扭曲的双起始左手双螺旋结构。我们建立了一个与 H5 结合的染色质纤维(包括一个完整的染色质体)的原子结构模型,该模型提供了全长连接组蛋白 H5 的结构细节,包括其 N 端结构域和一个类似 HMG-motif 的 C 端结构域。染色质结构显示,H5通过染色质纤维中的三接触模式与核小体偏离染色质结合。更重要的是,H5-染色质结构为核小体内和核小体间的相互作用提供了精细的分子基础。此外,我们通过在酿酒酵母中进行一系列遗传和基因组研究以及体外生物物理实验,系统地验证了四核体单元的生理功能和结构特征。此外,我们的结构揭示了组蛋白尾部的多种结构不对称性赋予了染色质纤维极性。这些发现从结构和机理上揭示了核糖体阵列如何在体外和体内折叠成具有极性的高阶染色质纤维。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Research
生物-细胞生物学
CiteScore
53.90
自引率
0.70%
发文量
2420
审稿时长
2.3 months
期刊介绍:
Cell Research (CR) is an international journal published by Springer Nature in partnership with the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences (CAS). It focuses on publishing original research articles and reviews in various areas of life sciences, particularly those related to molecular and cell biology. The journal covers a broad range of topics including cell growth, differentiation, and apoptosis; signal transduction; stem cell biology and development; chromatin, epigenetics, and transcription; RNA biology; structural and molecular biology; cancer biology and metabolism; immunity and molecular pathogenesis; molecular and cellular neuroscience; plant molecular and cell biology; and omics, system biology, and synthetic biology. CR is recognized as China's best international journal in life sciences and is part of Springer Nature's prestigious family of Molecular Cell Biology journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


