SMYD5 is a ribosomal methyltransferase that catalyzes RPL40 lysine methylation to enhance translation output and promote hepatocellular carcinoma
IF 28.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
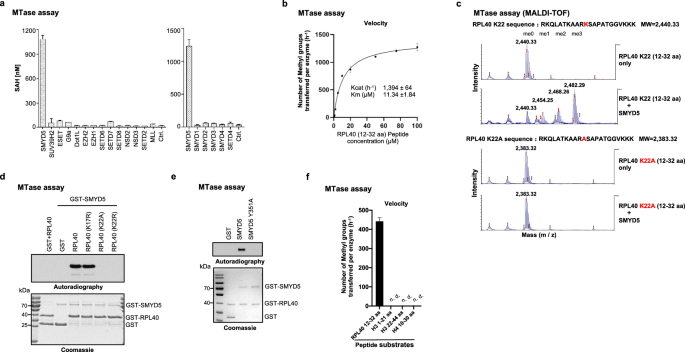
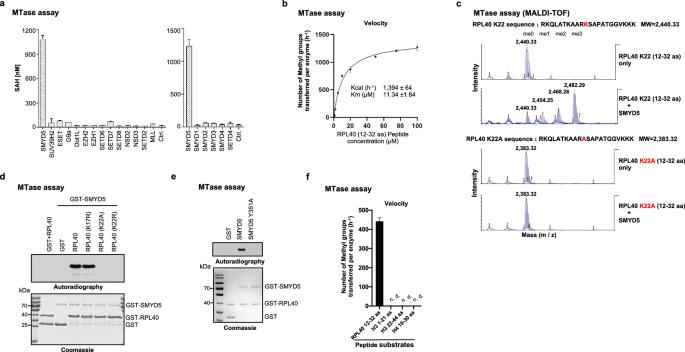
While lysine methylation is well-known for regulating gene expression transcriptionally, its implications in translation have been largely uncharted. Trimethylation at lysine 22 (K22me3) on RPL40, a core ribosomal protein located in the GTPase activation center, was first reported 27 years ago. Yet, its methyltransferase and role in translation remain unexplored. Here, we report that SMYD5 has robust in vitro activity toward RPL40 K22 and primarily catalyzes RPL40 K22me3 in cells. The loss of SMYD5 and RPL40 K22me3 leads to reduced translation output and disturbed elongation as evidenced by increased ribosome collisions. SMYD5 and RPL40 K22me3 are upregulated in hepatocellular carcinoma (HCC) and negatively correlated with patient prognosis. Depleting SMYD5 renders HCC cells hypersensitive to mTOR inhibition in both 2D and 3D cultures. Additionally, the loss of SMYD5 markedly inhibits HCC development and growth in both genetically engineered mouse and patient-derived xenograft (PDX) models, with the inhibitory effect in the PDX model further enhanced by concurrent mTOR suppression. Our findings reveal a novel role of the SMYD5 and RPL40 K22me3 axis in translation elongation and highlight the therapeutic potential of targeting SMYD5 in HCC, particularly with concurrent mTOR inhibition. This work also conceptually broadens the understanding of lysine methylation, extending its significance from transcriptional regulation to translational control.
SMYD5 是一种核糖体甲基转移酶,可催化 RPL40 赖氨酸甲基化,从而提高翻译输出并促进肝细胞癌的发生。
众所周知,赖氨酸甲基化是基因表达的转录调控因子,但它对翻译的影响却鲜为人知。位于 GTPase 激活中心的核心核糖体蛋白 RPL40 上赖氨酸 22 的三甲基化(K22me3)于 27 年前首次被报道。然而,它的甲基转移酶和在翻译中的作用仍未被探索。在这里,我们报告了 SMYD5 对 RPL40 K22 具有强大的体外活性,并在细胞中主要催化 RPL40 K22me3。SMYD5 和 RPL40 K22me3 的缺失会导致翻译输出的减少和延伸的紊乱,核糖体碰撞的增加就是证明。SMYD5和RPL40 K22me3在肝细胞癌(HCC)中上调,并与患者的预后呈负相关。在二维和三维培养中,消耗 SMYD5 会使 HCC 细胞对 mTOR 抑制剂不敏感。此外,在基因工程小鼠和患者异种移植(PDX)模型中,SMYD5 的缺失会明显抑制 HCC 的发育和生长,同时抑制 mTOR 会进一步增强 PDX 模型中的抑制作用。我们的研究结果揭示了 SMYD5 和 RPL40 K22me3 轴在翻译伸长中的新作用,并强调了靶向 SMYD5 在 HCC 中的治疗潜力,尤其是在同时抑制 mTOR 的情况下。这项研究还从概念上拓宽了人们对赖氨酸甲基化的认识,将其意义从转录调控扩展到了翻译调控。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Research
生物-细胞生物学
CiteScore
53.90
自引率
0.70%
发文量
2420
审稿时长
2.3 months
期刊介绍:
Cell Research (CR) is an international journal published by Springer Nature in partnership with the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences (CAS). It focuses on publishing original research articles and reviews in various areas of life sciences, particularly those related to molecular and cell biology. The journal covers a broad range of topics including cell growth, differentiation, and apoptosis; signal transduction; stem cell biology and development; chromatin, epigenetics, and transcription; RNA biology; structural and molecular biology; cancer biology and metabolism; immunity and molecular pathogenesis; molecular and cellular neuroscience; plant molecular and cell biology; and omics, system biology, and synthetic biology. CR is recognized as China's best international journal in life sciences and is part of Springer Nature's prestigious family of Molecular Cell Biology journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


