Syndecan-4 inhibition attenuates cartilage degeneration in temporomandibular joint osteoarthritis
Abstract
Background
Syndecan 4 (SDC4), a type I transmembrane proteoglycan, serves as a critical link between chondrocytes and the extracellular matrix.
Objective
This study aimed to explore the role of SDC4 in cartilage degeneration of temporomandibular joint osteoathritis (TMJOA).
Methods
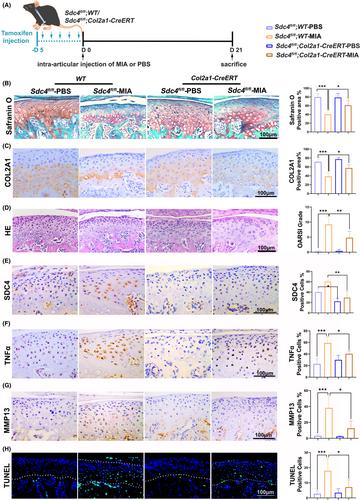
Condylar chondrocytes were stimulated with varying concentrations of recombinant rat interleukin-1β (rrIL-1β) and SDC4 small interfering RNA (si-SDC4). Anti-SDC4 ectodomain-specific antibodies or IgG were intra-articularly administrated in a TMJOA model rats. SDC4 conditional knockout (SDC4-cKO) and Sdc4flox/flox mice were induced TMJOA. Cartilage degeneration was assessed using haematoxylin & eosin (H&E) and safranin O (SO) staining. Protein levels of SDC4, matrix metalloproteinases (MMPs), a disintegrin and metalloproteinase with a thrombospondin motifs 5 (ADAMTS5), tumour necrosis factor α (TNFα), type II collagen (Col-II), aggrecan (ACAN), cleaved caspase 3 (CASP3), Ki67 and related pathways in condylar cartilage were evaluated by immunohistochemical (IHC) staining or western blot assays.
Results
SDC4 expression was evidently increased in MIA-model animals compared to control groups. rrIL-1β stimulation increased the expression of SDC4, MMP3 and ADAMTS5 expression in chondrocytes, while decreasing the expression of Col-II. These effects were reversed by si-SDC4 in vitro. In vivo, SDC4 blockade reduced the death of chondrocytes and the loss of cartilage matrix, which was evidenced by increased expression of Col-II and ACAN, and a decrease in SDC4, MMP13 and cleaved-CASP3-positive cells. Furthermore, the protein levels of ACAN and Ki67 were elevated, and the ERK1/2 and P38 signalling pathways were activated following SDC4 inhibition.
Conclusions
SDC4 inhibition significantly ameliorates condylar cartilage degeneration, which was mediated, at least partly, through P38 and ERK1/2 signalling. Inhibition of SDC4 may be of great value for the treatment of TMJOA.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


