Modulating regioselectivity of CYP107J3-catalyzed isophorone hydroxylation by disrupting the hydrophobic balance of the substrate binding pocket
引用次数: 0
Abstract
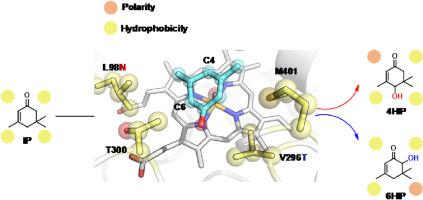
4-Hydroxyisophorone (4HIP) is an oxygenated intermediate derived from isophorone, serving as an important flavor and fragrance and chiral synthon of pharmaceutical drugs. In this study, a newly identified P450CYP107J3 from was found to prefer 4-hydroxylation of isophorone (80% regioselectivity) with 4HIP as the predominant product (59% product proportion). Bioinformatic analyses, including homologous modelling and molecular docking, reveal that four potential “hotspot” residues (L98, T300, M401 and V296) located on both sides of the substrate in the active pocket may control regioselectivity. By introducing polar residues to these hotspots to disrupt the hydrophobic balance, significant improvement in C4-regioselectivity was achieved by variants of L98, T300, and M401, with the most effective L98N exhibiting a notable enhancement of 93% C4-regioselectivity including 81% of 4HIP. Following, the double mutant L98N-M401F further improved both C4-regioselectivity (97%) and the proportion of 4HIP (86%). Remarkably, variant V296T essentially shifted regioselectivity from 4HIP to 6-hydroxyisophorone (6HIP) with an 80% preference for the latter, indicating the crucial role of V296 in controlling regioselectivity. Subsequently, the mechanism of regioselectivity of isophorone hydroxylation catalyzed by CYP107J3 was revealed by computational analysis. Furthermore, we demonstrated the generality of conserved hotspots L98 and V296 in mediating regioselectivity control in the CYP107J family members like CYP107J1 and CYP107J5. Overall, our study not only expands the biocatalytic toolbox for producing 4HIP and the -hydroxy ketone 6HIP but also provides efficient engineering strategy and knowledge for the regioselectivity control of P450s in potential applications.
通过破坏底物结合袋的疏水平衡调节 CYP107J3 催化的异佛尔酮羟基化的区域选择性
4-羟基异佛尔酮(4HIP)是异佛尔酮衍生的含氧中间体,是一种重要的香精香料和药物手性合成物。本研究发现,新发现的 P450CYP107J3 偏好异佛尔酮的 4- 羟基化(80% 的区域选择性),4HIP 是主要产物(产物比例为 59%)。包括同源建模和分子对接在内的生物信息学分析表明,位于活性袋底物两侧的四个潜在 "热点 "残基(L98、T300、M401 和 V296)可能控制着区域选择性。通过在这些热点残基上引入极性残基以破坏疏水平衡,L98、T300 和 M401 的变体显著提高了 C4 区域选择性,其中最有效的 L98N 显著提高了 93% 的 C4 区域选择性,包括 81% 的 4HIP。随后,双突变体 L98N-M401F 进一步提高了 C4regi 选择性(97%)和 4HIP 的比例(86%)。值得注意的是,变体 V296T 从根本上将区域选择性从 4HIP 转移到了 6-羟基异佛尔酮(6HIP),对后者的偏好达到了 80%,这表明 V296 在控制区域选择性方面起着至关重要的作用。随后,我们通过计算分析揭示了 CYP107J3 催化异佛尔酮羟基化的区域选择性机制。此外,我们还证明了保守热点 L98 和 V296 在 CYP107J 家族成员(如 CYP107J1 和 CYP107J5)中介导区域选择性控制的普遍性。总之,我们的研究不仅扩展了生产 4HIP 和-羟基酮 6HIP 的生物催化工具箱,而且为潜在应用中 P450s 的区域选择性控制提供了有效的工程策略和知识。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


