Functionalization of Silica Nanoparticles for Tailored Interactions with Intestinal Cells and Chemical Modulation of Paracellular Permeability
IF 8.3
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
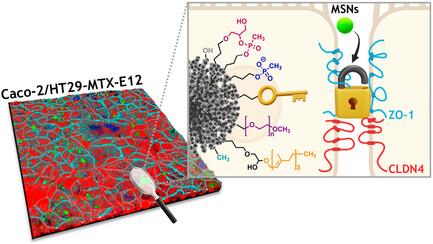
The intestinal compartment confines the gut microbiome while enabling food passage and absorption of active molecules. For the rational design of oral formulations aiming to overcome physiological barriers of the gut, it is crucial to understand how cells respond to the presence of nanoparticulate materials. Taking advantage of the versatility and biocompatibility of dendritic mesoporous silica nanoparticles (DMSNs), several post-grafting strategies are developed to diversify the surface properties of spherical DMSNs and then probe interactions with the intestinal coculture cell model Caco-2/HT29-MTX-E12. Herein, the functionalization of DMSNs with polyethylene glycol, phosphonate, methyl, and farnesol moieties enables the investigation of both particle penetration through the mucus layer and pathways relevant to intracellular uptake. Contributions of surface chemistry, charge, and colloidal stability are correlated with the modulation of particle movement through the mucus and the organization of cell–cell junctions. Hydrophilic and negative functionalities favor particle distribution toward the intestinal monolayer. Instead, hydrophobic DMSNs are hindered by the mucus, possibly limiting cell contact. Hybrid surfaces, combining phosphonate and long carbon chain functions, support diffusion through the mucus and foster the paracellular permeability as well as the transient barrier relapse, as indicated by increased cell–cell distances and reorganization of tight junctions.
功能化二氧化硅纳米粒子,实现与肠道细胞的定制互动以及对细胞旁渗透性的化学调节
肠道隔间在保证食物通过和活性分子吸收的同时,也限制了肠道微生物群。为了合理设计旨在克服肠道生理障碍的口服制剂,了解细胞如何对纳米颗粒材料的存在做出反应至关重要。利用树枝状介孔二氧化硅纳米颗粒(DMSNs)的多功能性和生物相容性,我们开发了几种后接枝策略,使球形 DMSNs 的表面性质多样化,然后探究其与肠道细胞模型 Caco-2/HT29-MTX-E12 的相互作用。在这里,用聚乙二醇、膦酸盐、甲基和法呢醇分子对 DMSNs 进行官能化,既能研究颗粒穿透粘液层的情况,也能研究与细胞内吸收相关的途径。表面化学、电荷和胶体稳定性的贡献与颗粒在粘液中的运动调节和细胞-细胞连接的组织有关。亲水性和负功能性有利于颗粒向肠单层分布。相反,疏水性 DMSN 会受到粘液的阻碍,可能会限制细胞接触。结合了膦酸盐和长碳链功能的混合表面支持通过粘液进行扩散,并通过增加细胞间距离和重组紧密连接来促进细胞旁渗透性和瞬时屏障复通。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
14.00
自引率
2.40%
发文量
0
期刊介绍:
Small Science is a premium multidisciplinary open access journal dedicated to publishing impactful research from all areas of nanoscience and nanotechnology. It features interdisciplinary original research and focused review articles on relevant topics. The journal covers design, characterization, mechanism, technology, and application of micro-/nanoscale structures and systems in various fields including physics, chemistry, materials science, engineering, environmental science, life science, biology, and medicine. It welcomes innovative interdisciplinary research and its readership includes professionals from academia and industry in fields such as chemistry, physics, materials science, biology, engineering, and environmental and analytical science. Small Science is indexed and abstracted in CAS, DOAJ, Clarivate Analytics, ProQuest Central, Publicly Available Content Database, Science Database, SCOPUS, and Web of Science.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


