Cross-Species Applications of Peptide Substrate Reporters to Quantitative Measurements of Kinase Activity
IF 4.6
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
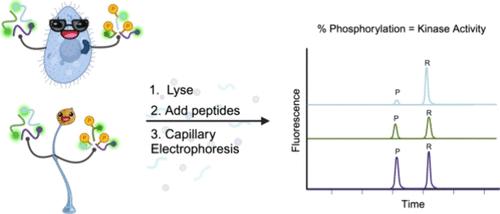
Peptide substrate reporters are short chains of amino acids designed to act as substrates for enzymes of interest. Combined with capillary electrophoresis and laser-induced fluorescence detection (CE-LIF), they are powerful molecular tools for quantitative measurements of enzyme activity even at the level of single cells. Although most peptide substrate reporters have been optimized for human or murine cells in health-related applications, their performance in nonmammalian organisms remains largely unexplored. In this study, we evaluated three peptide substrate reporters for protein kinase B (PKB) in two eukaryotic microbes, Dictyostelium discoideum and Tetrahymena thermophila, which are evolutionarily distant from mammals and from each other yet express PKB homologues. All three peptide substrate reporters were phosphorylated in lysates from both organisms but with varying phosphorylation kinetics and stability. To demonstrate reporter utility, we used one to screen for and identify the previously unknown stimulus needed to activate PHK5, the PKB homologue in T. thermophila. In D. discoideum, we employed the highly quantitative nature of these assays using CE-LIF to make precise measurements of PKB activity in response to transient stimulation, drug treatment, and genetic mutation. These results underscore the broad applicability of peptide substrate reporters across diverse species while highlighting the need for further research to determine effective peptide stabilization strategies across different biological contexts.
多肽底物报告器在激酶活性定量测量中的跨物种应用
多肽底物报告物是由氨基酸组成的短链,可作为相关酶的底物。它们与毛细管电泳和激光诱导荧光检测(CE-LIF)相结合,是即使在单细胞水平上也能定量测量酶活性的强大分子工具。虽然大多数肽底物报告物都已针对人类或鼠类细胞的健康相关应用进行了优化,但它们在非哺乳动物中的性能在很大程度上仍未得到探索。在这项研究中,我们评估了两种真核微生物--盘基竹荪(Dictyostelium discoideum)和嗜热四膜虫(Tetrahymena thermophila)--中蛋白激酶 B(PKB)的三种多肽底物报告物。在这两种生物的裂解液中,所有三种肽底物报告物都发生了磷酸化,但磷酸化动力学和稳定性各不相同。为了证明报告物的效用,我们用其中一种报告物筛选并确定了激活嗜热菌中 PKB 同源物 PHK5 所需的未知刺激。在 Discoideum 中,我们利用 CE-LIF 这些检测方法的高度定量性,精确测量了 PKB 在瞬时刺激、药物治疗和基因突变下的活性。这些结果凸显了多肽底物报告物在不同物种中的广泛适用性,同时也强调了在不同生物环境中确定有效多肽稳定策略的进一步研究的必要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Measurement Science Au
化学计量学-
CiteScore
5.20
自引率
0.00%
发文量
0
期刊介绍:
ACS Measurement Science Au is an open access journal that publishes experimental computational or theoretical research in all areas of chemical measurement science. Short letters comprehensive articles reviews and perspectives are welcome on topics that report on any phase of analytical operations including sampling measurement and data analysis. This includes:Chemical Reactions and SelectivityChemometrics and Data ProcessingElectrochemistryElemental and Molecular CharacterizationImagingInstrumentationMass SpectrometryMicroscale and Nanoscale systemsOmics (Genomics Proteomics Metabonomics Metabolomics and Bioinformatics)Sensors and Sensing (Biosensors Chemical Sensors Gas Sensors Intracellular Sensors Single-Molecule Sensors Cell Chips Arrays Microfluidic Devices)SeparationsSpectroscopySurface analysisPapers dealing with established methods need to offer a significantly improved original application of the method.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


