Improvement of Asymmetric Reactions via Remote Electronic Tuning of N-Heterocyclic Carbene Catalysts
IF 3.8
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Aminoindanol-derived N-heterocyclic carbene catalysts have been widely used to achieve highly enantioselective reactions. Recently, substitution at the indane moiety of the catalysts has been found to have a great impact on the outcome of some reactions, even though the substituted position is distant from the carbene catalytic center. Especially, the introduction of an electron-withdrawing group improves the yield and enantioselectivity. This review provides an overview of recent reports on the improvement of N-heterocyclic-carbene-catalyzed asymmetric reactions by introducing electron-withdrawing remote substituents.
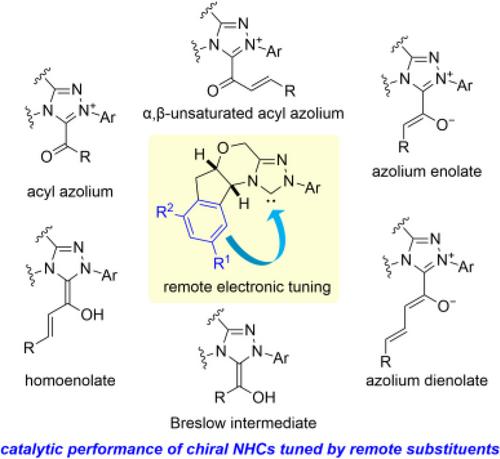
通过 N-杂环羰基催化剂的远程电子调谐改善不对称反应
氨基茚醇衍生的 N-杂环碳烯催化剂已被广泛用于实现高对映体选择性反应。最近,人们发现催化剂的茚基取代对某些反应的结果有很大影响,即使取代位置远离碳催化中心。特别是,引入一个抽电子基团可提高产率和对映体选择性。本综述概述了最近关于通过引入远距离取电子取代基改善 N-杂环碳烯催化的不对称反应的报道。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
ChemCatChem
化学-物理化学
CiteScore
8.10
自引率
4.40%
发文量
511
审稿时长
1.3 months
期刊介绍:
With an impact factor of 4.495 (2018), ChemCatChem is one of the premier journals in the field of catalysis. The journal provides primary research papers and critical secondary information on heterogeneous, homogeneous and bio- and nanocatalysis. The journal is well placed to strengthen cross-communication within between these communities. Its authors and readers come from academia, the chemical industry, and government laboratories across the world. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies, and is supported by the German Catalysis Society.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


