Antifungal heteroresistance causes prophylaxis failure and facilitates breakthrough Candida parapsilosis infections
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
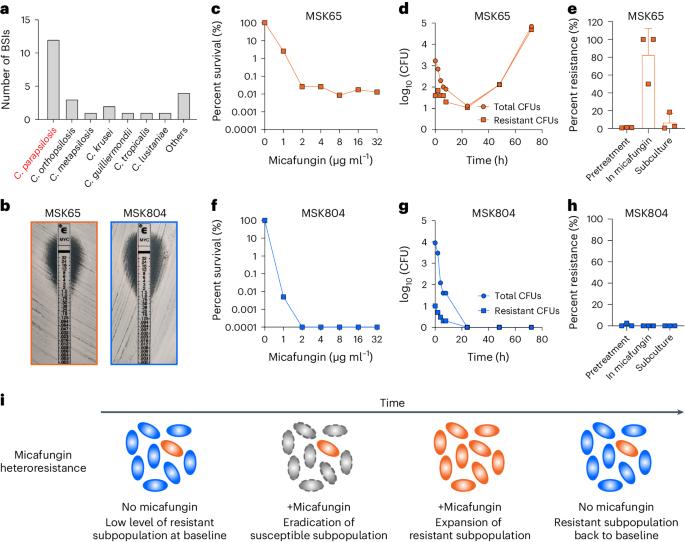
Breakthrough fungal infections in patients on antimicrobial prophylaxis during allogeneic hematopoietic cell transplantation (allo-HCT) represent a major and often unexplained cause of morbidity and mortality. Candida parapsilosis is a common cause of invasive candidiasis and has been classified as a high-priority fungal pathogen by the World Health Organization. In high-risk allo-HCT recipients on micafungin prophylaxis, we show that heteroresistance (the presence of a phenotypically unstable, low-frequency subpopulation of resistant cells (~1 in 10,000)) underlies breakthrough bloodstream infections by C. parapsilosis. By analyzing 219 clinical isolates from North America, Europe and Asia, we demonstrate widespread micafungin heteroresistance in C. parapsilosis. Standard antimicrobial susceptibility tests, such as broth microdilution or gradient diffusion assays, which guide drug selection for invasive infections, fail to detect micafungin heteroresistance in C. parapsilosis. To facilitate rapid detection of micafungin heteroresistance in C. parapsilosis, we constructed a predictive machine learning framework that classifies isolates as heteroresistant or susceptible using a maximum of ten genomic features. These results connect heteroresistance to unexplained antifungal prophylaxis failure in allo-HCT recipients and demonstrate a proof-of-principle diagnostic approach with the potential to guide clinical decisions and improve patient care. Bloodstream infections with Candida parapsilosis in allogeneic hematopoietic cell transplantation recipients are associated with heteroresistance to the antifungal micafungin, which can be predicted from genomic features using machine learning.

抗真菌异抗性导致预防失败,并助长了副丝状念珠菌的突破性感染
在异基因造血细胞移植(allo-HCT)过程中,接受抗菌预防治疗的患者发生突破性真菌感染是导致发病和死亡的一个主要原因,而且往往是无法解释的原因。副丝状念珠菌是侵袭性念珠菌病的常见病因,已被世界卫生组织列为高度优先的真菌病原体。在接受米卡芬净预防治疗的高风险异体肝移植受者中,我们发现异种抗性(存在表型不稳定、低频率的抗性细胞亚群(约万分之一))是副丝状念珠菌突破性血流感染的基础。通过分析来自北美、欧洲和亚洲的 219 个临床分离株,我们证明了副丝状菌中广泛存在的米卡芬净异抗性。肉汤微量稀释法或梯度扩散法等标准抗菌药敏感性测试可指导对侵入性感染的用药选择,但却无法检测出副丝状菌对米卡芬净的异抗性。为了便于快速检测副丝状菌中的米卡芬净异抗性,我们构建了一个预测性机器学习框架,利用最多十个基因组特征将分离物分为异抗性和易感性。这些结果将异种抗药性与异体肝移植受者中无法解释的抗真菌预防失败联系起来,证明了一种原理性诊断方法具有指导临床决策和改善患者护理的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


