Targeting axonal guidance dependencies in glioblastoma with ROBO1 CAR T cells
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
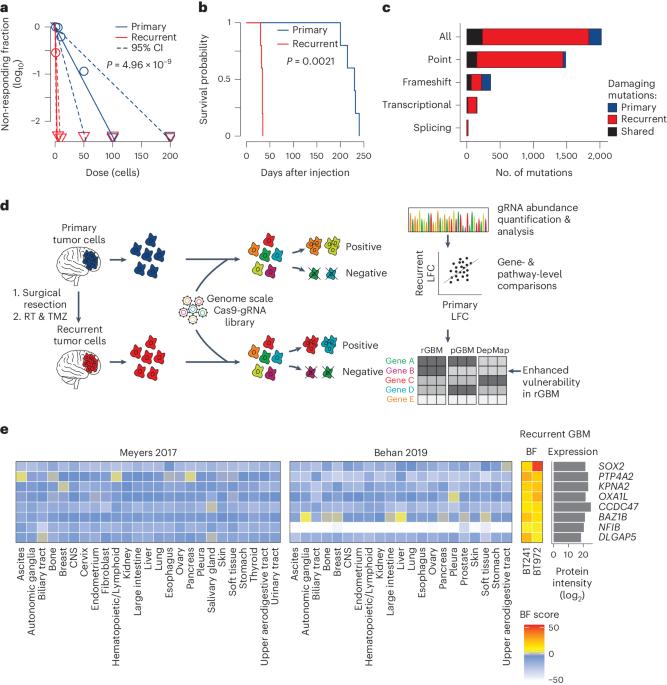
Resistance to genotoxic therapies and tumor recurrence are hallmarks of glioblastoma (GBM), an aggressive brain tumor. In this study, we investigated functional drivers of post-treatment recurrent GBM through integrative genomic analyses, genome-wide genetic perturbation screens in patient-derived GBM models and independent lines of validation. Specific genetic dependencies were found consistent across recurrent tumor models, accompanied by increased mutational burden and differential transcript and protein expression compared to its primary GBM predecessor. Our observations suggest a multi-layered genetic response to drive tumor recurrence and implicate PTP4A2 (protein tyrosine phosphatase 4A2) as a modulator of self-renewal, proliferation and tumorigenicity in recurrent GBM. Genetic perturbation or small-molecule inhibition of PTP4A2 acts through a dephosphorylation axis with roundabout guidance receptor 1 (ROBO1) and its downstream molecular players, exploiting a functional dependency on ROBO signaling. Because a pan-PTP4A inhibitor was limited by poor penetrance across the blood–brain barrier in vivo, we engineered a second-generation chimeric antigen receptor (CAR) T cell therapy against ROBO1, a cell surface receptor enriched across recurrent GBM specimens. A single dose of ROBO1-targeted CAR T cells doubled median survival in cell-line-derived xenograft (CDX) models of recurrent GBM. Moreover, in CDX models of adult lung-to-brain metastases and pediatric relapsed medulloblastoma, ROBO1 CAR T cells eradicated tumors in 50–100% of mice. Our study identifies a promising multi-targetable PTP4A–ROBO1 signaling axis that drives tumorigenicity in recurrent GBM, with potential in other malignant brain tumors. Functional CRISPR screens in patient-matched pre-treatment and post-treatment glioblastoma models identify the PTP4A–ROBO1 axis as a driver of tumorigenicity and enriched ROBO1 expression in recurrent glioblastoma that can be targeted with CAR T cell therapy.
用ROBO1 CAR T细胞靶向胶质母细胞瘤的轴突导向依赖性
对基因毒性疗法的抗药性和肿瘤复发是侵袭性脑肿瘤胶质母细胞瘤(GBM)的特征。在本研究中,我们通过综合基因组分析、患者衍生 GBM 模型的全基因组遗传扰动筛选和独立验证,研究了治疗后复发 GBM 的功能驱动因素。与原发性 GBM 的前身相比,复发性肿瘤模型中的特定遗传依赖性伴随着突变负荷的增加以及转录本和蛋白质表达的差异。我们的观察结果表明,驱动肿瘤复发的基因反应是多层次的,并表明 PTP4A2(蛋白酪氨酸磷酸酶 4A2)是复发性 GBM 自我更新、增殖和致瘤性的调节因子。基因扰动或小分子抑制 PTP4A2 的作用是通过与迂回引导受体 1(ROBO1)及其下游分子角色的去磷酸化轴,利用 ROBO 信号的功能依赖性。由于泛PTP4A抑制剂在体内对血脑屏障的穿透性较差,我们设计了一种针对ROBO1的第二代嵌合抗原受体(CAR)T细胞疗法,ROBO1是复发性GBM标本中富含的一种细胞表面受体。在复发性 GBM 的细胞系衍生异种移植(CDX)模型中,单剂量 ROBO1 靶向 CAR T 细胞可使中位生存期延长一倍。此外,在成人肺脑转移瘤和小儿复发性髓母细胞瘤的 CDX 模型中,ROBO1 CAR T 细胞根除了 50-100% 小鼠的肿瘤。我们的研究发现了一种有前景的多靶点 PTP4A-ROBO1 信号轴,它能驱动复发性 GBM 的致瘤性,在其他恶性脑肿瘤中也有应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


