Safety and reactogenicity of a controlled human infection model of sand fly-transmitted cutaneous leishmaniasis
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The leishmaniases are globally important parasitic diseases for which no human vaccines are currently available. To facilitate vaccine development, we conducted an open-label observational study to establish a controlled human infection model (CHIM) of sand fly-transmitted cutaneous leishmaniasis (CL) caused by Leishmania major. Between 24 January and 12 August 2022, we exposed 14 participants to L. major-infected Phlebotomus duboscqi. The primary objective was to demonstrate effectiveness of lesion development (take rate) and safety (absence of CL lesion at 12 months). Secondary and exploratory objectives included rate of lesion development, parasite load and analysis of local immune responses by immunohistology and spatial transcriptomics. Lesion development was terminated by therapeutic biopsy (between days 14 and 42 after bite) in ten participants with clinically compatible lesions, one of which was not confirmed by parasite detection. We estimated an overall take rate for CL development of 64% (9/14). Two of ten participants had one and one of ten participants had two lesion recurrences 4–8 months after biopsy that were treated successfully with cryotherapy. No severe or serious adverse events were recorded, but as expected, scarring due to a combination of CL and the biopsy procedure was evident. All participants were lesion free at >12-month follow-up. We provide the first comprehensive map of immune cell distribution and cytokine/chemokine expression in human CL lesions, revealing discrete immune niches. This CHIM offers opportunities for vaccine candidate selection based on human efficacy data and for a greater understanding of immune-mediated pathology. ClinicalTrials.gov identifier: NCT04512742 . The first controlled human infection model of cutaneous leishmaniasis transmitted by sand flies enables assessment of immune responses and parasitism in infected participants that can inform future vaccine and therapeutic development.
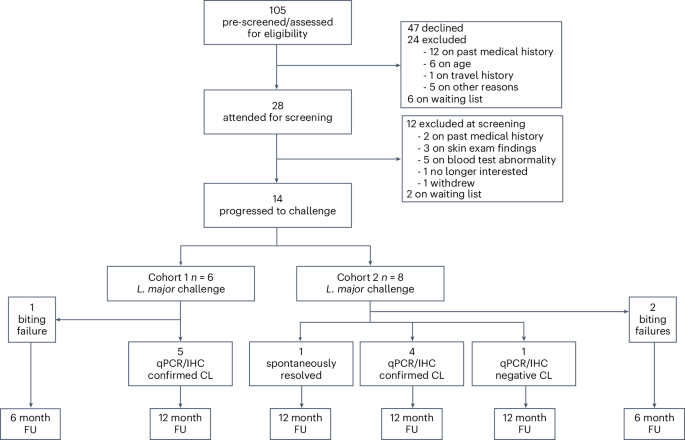
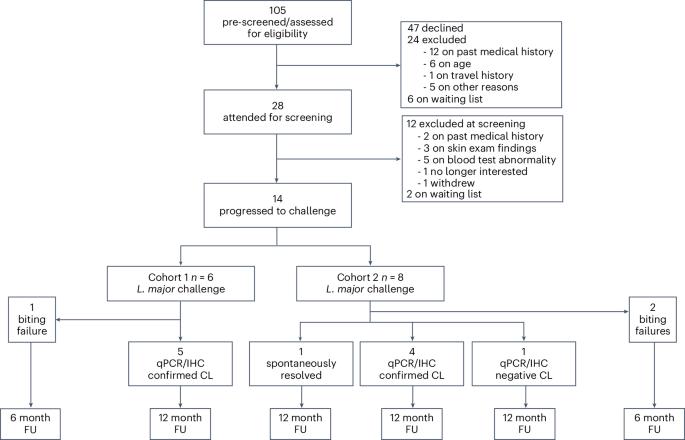
沙蝇传播皮肤利什曼病人类受控感染模型的安全性和反应性
利什曼病是全球重要的寄生虫病,目前尚无人类疫苗可用。为了促进疫苗的开发,我们开展了一项开放标签观察研究,以建立由大利什曼原虫引起的沙蝇传播皮肤利什曼病(CL)的受控人类感染模型(CHIM)。在2022年1月24日至8月12日期间,我们让14名参与者接触了感染大利什曼病菌的沙蝇。首要目标是证明病变发展的有效性(感染率)和安全性(12 个月后无 CL 病变)。次要和探索性目标包括病变发展率、寄生虫载量以及通过免疫组织学和空间转录组学分析局部免疫反应。通过对 10 名参与者进行治疗性活检(咬伤后第 14 到 42 天之间)来终止病变的发展,这些参与者的病变与临床症状相符,其中一人未通过寄生虫检测得到证实。我们估计,CL发展的总体成功率为 64%(9/14)。10 名参与者中,有 2 人在活检后 4-8 个月复发 1 次,1 人在活检后 4-8 个月复发 2 次,均成功接受了冷冻疗法。没有严重不良事件的记录,但正如预期的那样,CL 和活检术共同造成的疤痕是显而易见的。所有参与者在>12个月的随访中均无病变。我们首次提供了人类CL病变中免疫细胞分布和细胞因子/趋化因子表达的综合图谱,揭示了离散的免疫龛位。该CHIM为根据人类疗效数据选择候选疫苗提供了机会,也为进一步了解免疫介导的病理学提供了机会。ClinicalTrials.gov 标识符:NCT04512742。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


